chem whatarethemechanismsofchemicalchange
Reactivity 3.4.9 - nucleophilic substitution reactions include the reactions between halogenoalkanes and nucleophiles
Reactivity 3.4.10 - the rate of the substitution reactions is influenced by the identity of the leaving group
see 3.2.7 stereoisomers (HL)
see 2.2.2 collision theory
see 2.2.6, 2.2.7 and 2.2.8 reaction mechanisms
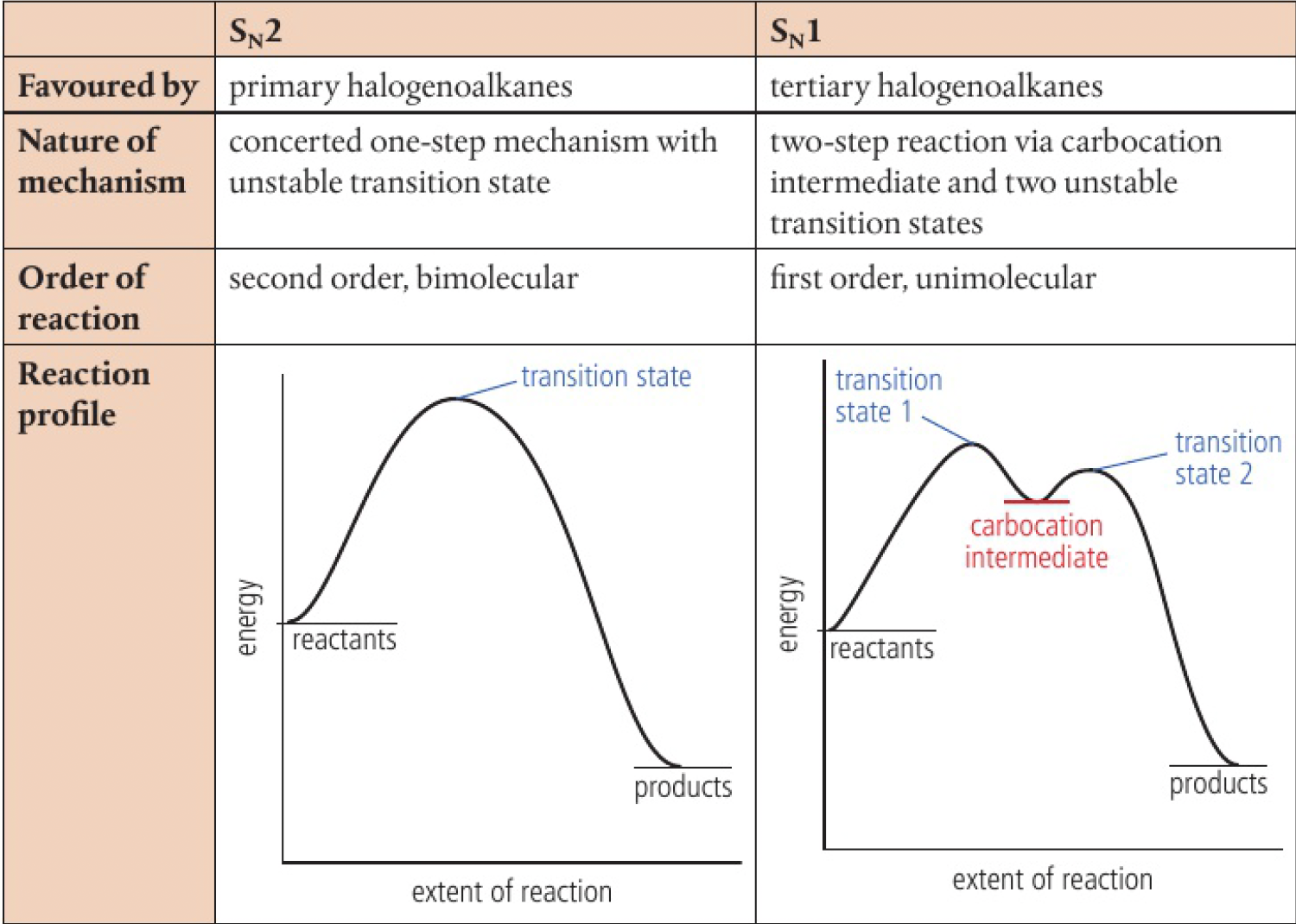
- substitution nucleophilic, is used to describe the reactions of nucleophiles with primary and tertiary halogenoalkanes - the exact mechanism of these reactions depends on whether the halogenoalkane is primary, secondary, or tertiary
mechanism
primary halogenoalkanes react via
- primary halogenoalkanes have at least 2 hydrogen atoms attached to the carbon of the
bond. for example:
[H]C([H])([H])C([H])([H])Clthe overall reaction that occurs with
- as the hydrogen atoms are so small, the carbon atom is relatively open to attack by the nucleophile
- an unstable transition state is formed in which the carbon is weakly bonded to both the halogen and the nucleophile
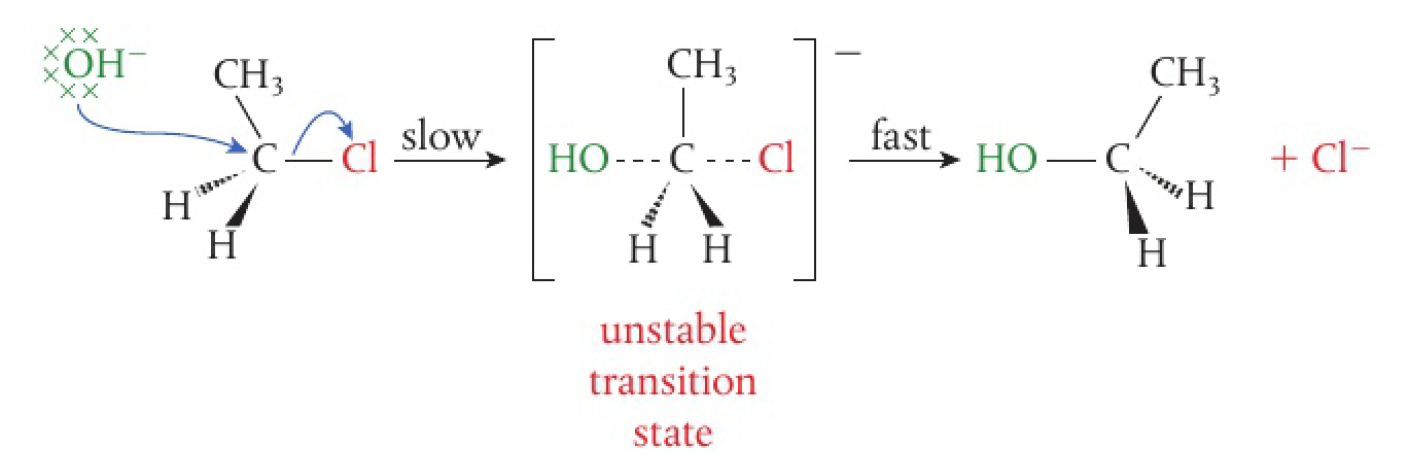
- this is a one-step concerted reaction with an unstable transition state
- concerted means that the bonds break and form at the same time
- since the mechanism is dependent on the concentration of both of
and , it is a bimolecular reaction, and will be second order overall
therefore, this mechanism is fully described as
the nucleophile attacks the electrophilic carbon atom on the opposite side from the leaving group, which causes an inversion of the arrangement of the atoms around the carbon atom.
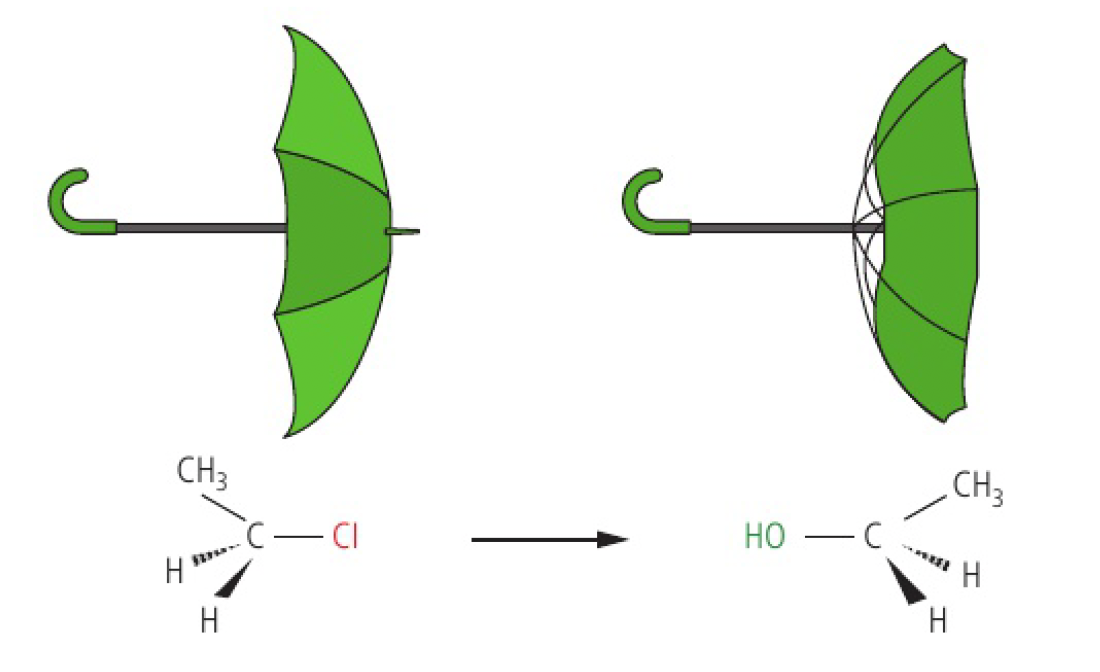
these configurations are a result of different spatial arrangements around tetrahedrally bonded carbon atoms.
the
- bond formation comes before bond cleavage in the transition state, so the stereochemistry of the carbon attacked is not lost
- optically active reactants will react via
to give optically active products.
tertiary halogenoalkanes react via
- tertiary halogenoalkanes have 3 alkyl groups attached to the carbon of the
bond. eg:
CC(C)(C)Cl-
here, the presence of three alkyl groups around the carbon of the
bond causes what is called steric hindrance -
the presence of the bulky groups makes it difficult for an incoming group to attack the carbon atom
-
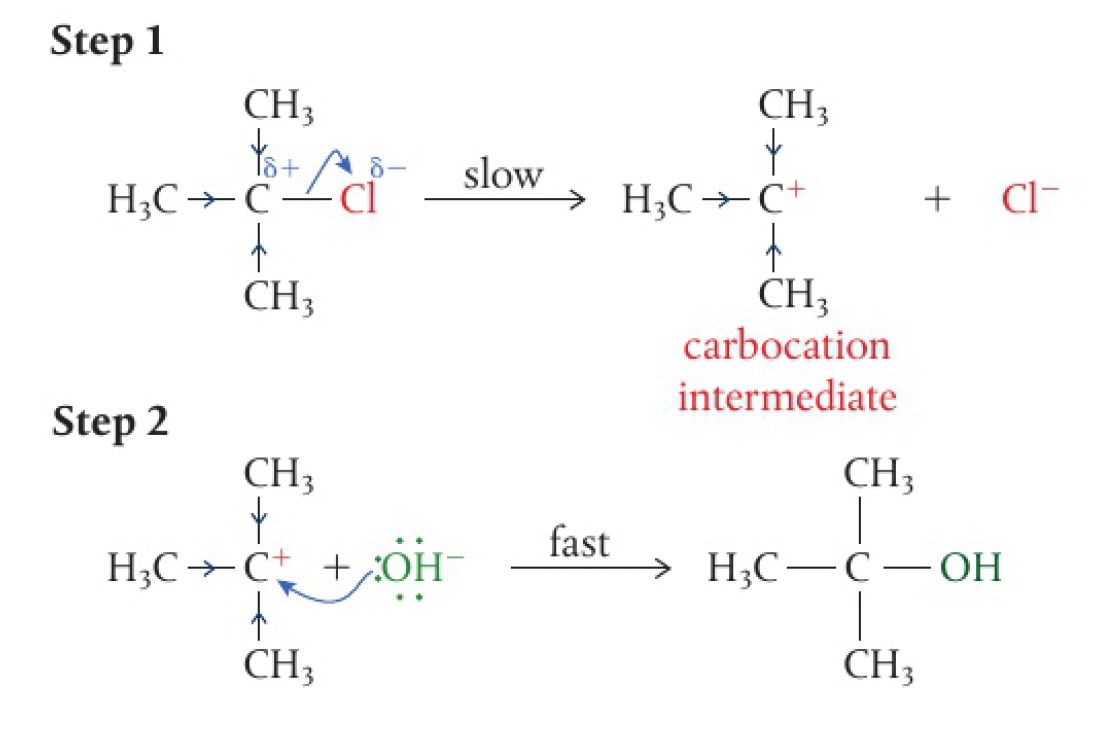
the first step of an
reaction involves heterolytic fission of the bond and the halide ion leaving -
this creates a carbocation intermediate with a positive charge centred on the carbon
-
this intermediate is then attacked by the nucleophilic
in the second step of the reaction, leading to the formation of a new bond -
the carbocation intermediate has a planar shape, which means the nucleophile can attack from any position in the second step
-
thus, the
reaction is not stereospecific, and optically active reactants react via to give products that are racemic mixtures -
this mechanism is favoured in tertiary halogenoalkanes as the carbocation is stabilised by the three alkyl groups
-
the three alkyl groups have a positive inductive effect, meaning that they are electron donating
-
this allows the carbocation to persist long enough for the second step to occur
the blue arrows on the bonds show this positive inductive effect
since the rate-determining step is only determined by the concentration of the
- therefore, this mechanism is fully described as
: substitution nucleophilic order
secondary halogenoalkanes
it is not possible to be precise about the mechanism of nucleophilic substitution in secondary halogenoalkanes, as data shows that they usually undergo a mixture of both
rate:
the influence of the leaving group
experimental data shows that the rate of nucleophilic reactions for halogenoalkanes with the same carbon chain length and branching, follows the order:
- this is consistent with the bond enthalpies of
bonds - the weaker the bond, the easier it is to break and the better the halide ion is as a leaving group
best to worst:
- the
mechanism is favoured by tertiary halogenoalkanes and is a two-step mechanism - the rate depends on the slow first step and it is first order with the rate only depending on the concentration of the halogenoalkane:
-
since there are two steps, it proceeds from reactants to products via an intermediate, which is shown as a local minimum in the reaction profile
-
because the
mechanism involves 2 steps, the reaction profile has 2 activation energies and each step proceeds through independent transition states
- the
mechanism is favoured by primary halogenoalkanes and occurs through a concerted one-step mechanism - it is a second order reaction with the rate depending on the concentrations of both the halogenoalkane and nucleophile:
- since there is only 1 step, it proceeds from reactants to products via a single transition state
summary and