Structure 3.2.9 - infrared spectra can be used to identify the type of bond present in a molecule
see 3.2.2 functional groups and classes of compounds
wavelength (
frequency (
in IR spectroscopy, the frequency is measured as the number of waves per centimetre - wavenumber (
reading
electromagnetic radiation can be used as a carrier of information:
- radio waves are used in NMR and gives information about the environment of atoms
- microwaves cause molecules to increase their rotation energy, giving information about bond lengths
- infrared radiation is absorbed by certain bonds causing them to stretch or bend, giving information about the bonds in a molecule
- visible light and ultraviolet light can produce electronic transitions and give information about the electronic energy levels in a molecule
- x-rays are produced when electrons make transitions between inner energy levels. they have wavelengths of the same order of magnitude as the inter-atomic distances in crystals and produce diffraction patterns which provide direct evidence of molecular and crystal structure
why are some molecules IR active?
a chemical bond vibrates and bends at a natural frequency, depending on the bond strength and masses of atoms. lighter atoms and multiple bonds vibrate at higher frequencies than heavier atoms and single bonds
simple diatomic molecules can only vibrate when the bond stretches, and only if it is polar.
- the electric field component of the electromagnetic wave interacts with the areas of partial positive and negative charge in a molecule, exciting the vibration energy of the molecule
- the change in vibration energy produces a change in the dipole moment of the molecule, the intensity of which depending on the polarity of the bond
- symmetrical non-polar bonds don’t absorb radiation, as they cannot interact with an electric field (eg
)
in polyatomic molecules, it is more correct to consider the molecule stretching and bending as a whole, rather than considering the individual bonds.
water can vibrate at 3 fundamental frequencies. since the three modes results in a change in the dipole of the molecule, they can be detected with IR spectroscopy.
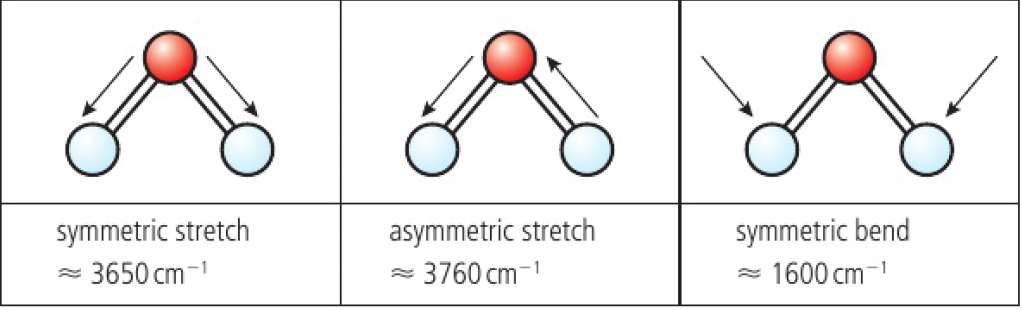
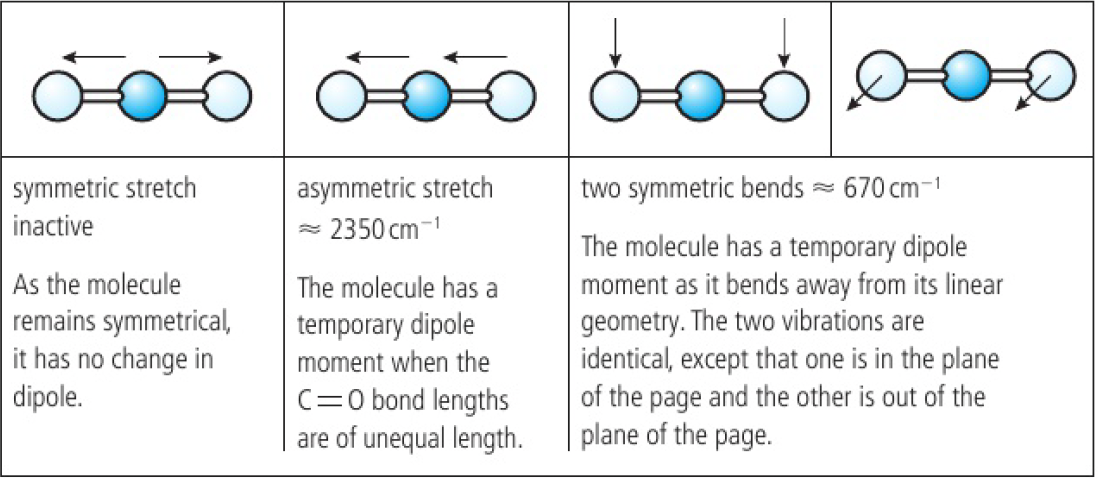
for a symmetrical linear molecule, there are 4 modes of vibration, however, the symmetric stretch is IR inactive as it produces no change in dipole moment. the dipoles of both
greenhouse effect
the temperature of Earth is maintained by a steady-state balance between the energy received from the Sun and the energy leaving the Earth and going into space.
most solar radiation passes through the atmosphere and warms the surface of the Earth. the warm Earth surface radiates some of this energy as longer wavelength infrared radiation, which is absorbed by greenhouse molecules. this makes the air warmer, causing it to radiate heat in return. some is reradiated back to the surface of the Earth and some into space.
the ability of a molecule to absorb infrared radiation depends on the change in dipole moment that occurs as it vibrates, so some greenhouse gases are much more potent than others.
the global warming potential of a greenhouse gas compares the amount of IR energy that one tonne of gas would absorb to the amount that would be absorbed by one tonne of
it depends on:
- how effective the individual gas molecules are at absorbing IR radiation
- the atmospheric lifetime of the gas (how long it stays in the atmosphere before being removed)
challenge questions
- calculate the energy of a photon of visible light with a frequency of
. express your answer in
- a bond has an IR absorption at
, calculate the wavelength of the radiation absorbed and the natural frequency of the bond.
wavenumber = wavelengths per centimetre
wavelength = centimetres per wavenumber
wavelength = 0.00047619cm
= 4762nm
frequency =
=
=