chem whatarethemechanismsofchemicalchange
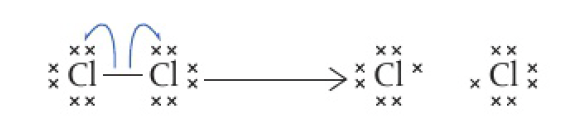
Reactivity 3.3.2 - radicals are produced by homolytic fission, eg.g. of halogens, in the presence of ultraviolet light or heat
see convention for depicting organic reaction mechanisms
- 2 equal radicals
- equal assignment of electrons
- endothermic process
thermolytic fission
- low energy
photolytic fission
- higher energy than thermolytic fission
chlorine radicals and ozone depletion
- chlorofluorocarbons (CFCs)
- used in aerosols, refrigerants, solvents, plastics
- low reactivity
- low toxicity in troposphere
entering the stratosphere, they are broken down through photolytic fission
dichlorodifluoromethane
[F]C([Cl])([Cl])[F]the chlorine radicals released catalyse the decomposition of ozone,
here
- chlorine radicals are particularly harmful as they can catalyse the decomposition thousands of ozone molecules
- chlorine radicals do not readily react with
challenge questions
- average bond enthalpies for the
apply this formula