chem whatarethemechanismsofchemicalchange
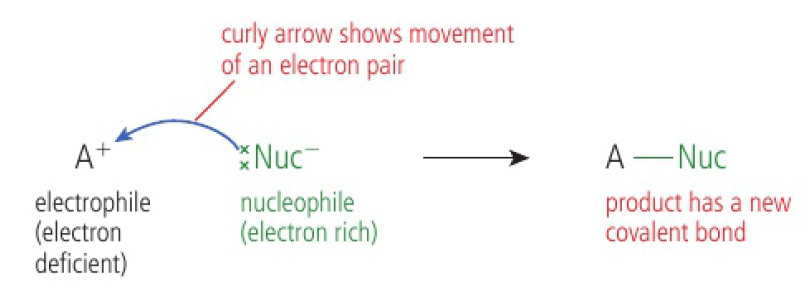
Reactivity 3.4.1 - a nucleophile is a reactant that forms a bond to its reaction partner (the electrophile) by donating both bonding electrons
nucleophiles:
- are a reactant that forms a covalent bond to its reaction partner (the electrophile) by donating both bonding electrons
- are typically electron rich
- have one or more lone pairs of electrons
- may carry a negative charge
eg:
- neutral:
- anions:
the covalent bond formed is a coordination bond
neutral molecules can be nucleophiles as they contain electron-rich areas that result from instantaneous dipoles or permanent dipoles