chem whatarethemechanismsofchemicalchange
Reactivity 3.2.6 - a primary (voltaic) cell is an electrochemical cell that converts energy from spontaneous redox reactions to electrical energy
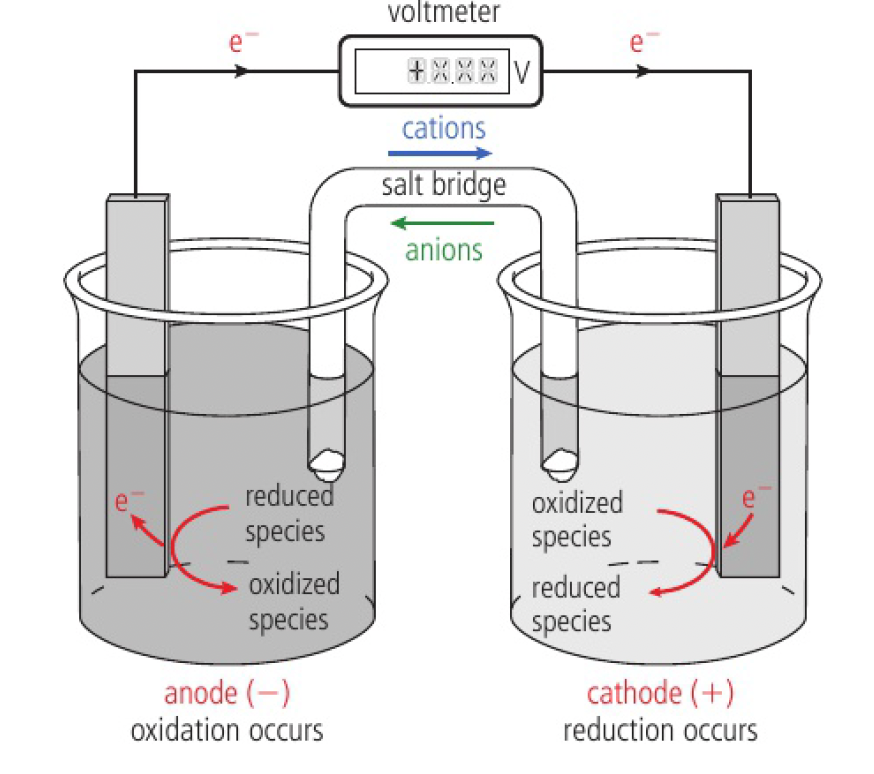
if half-reactions occur at spatially separated electrodes, the electrons need to pass through an external circuit between the electrodes and an electric current is produced
as the chemicals are not renewed during the process, it is known as a primary cell. a battery normally consists of several cells connected together.
example of a voltaic cell:
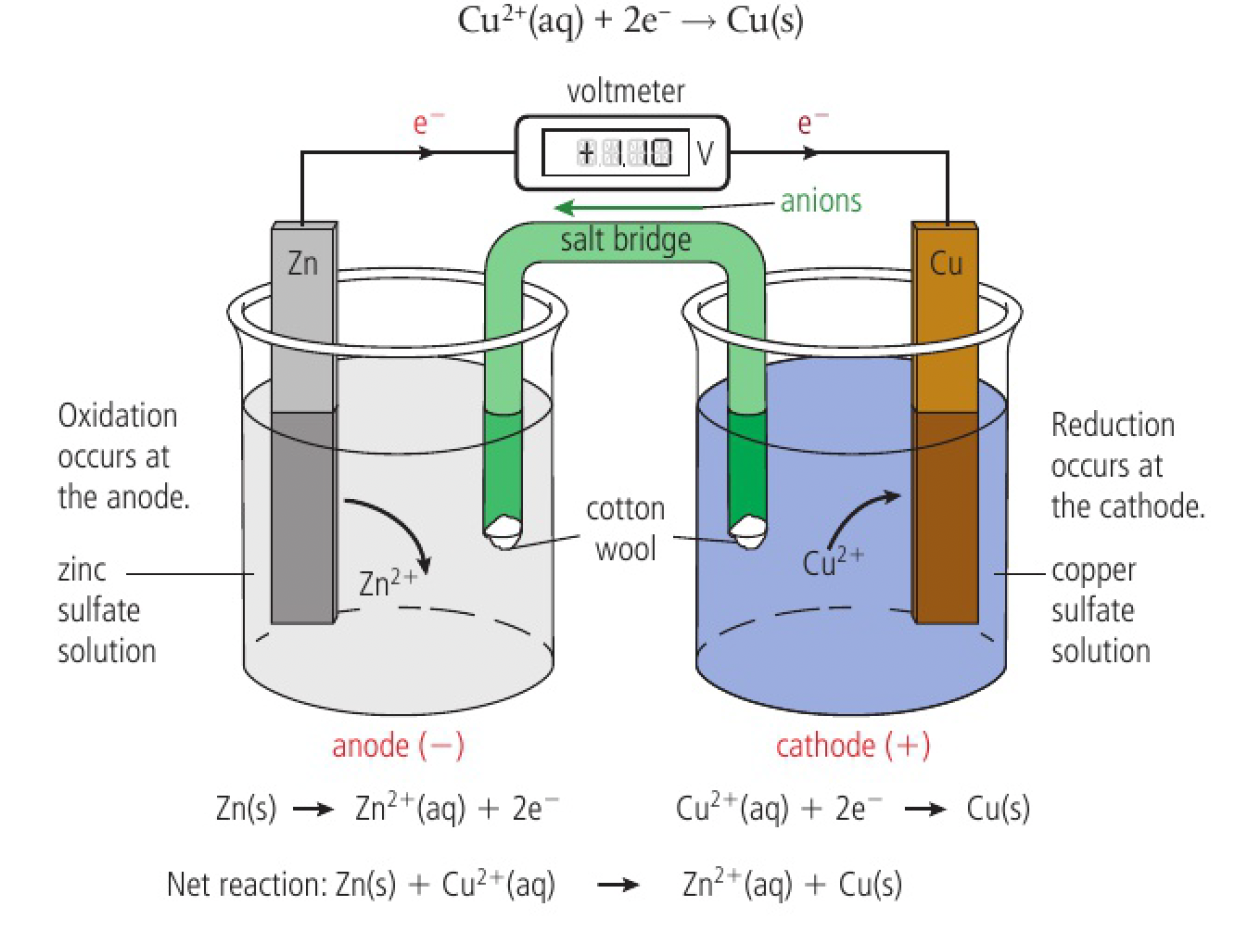
in a zinc half-cell, zinc atoms form ions by releasing electrons that make the surface of the metal negatively charged with respect to the solution.
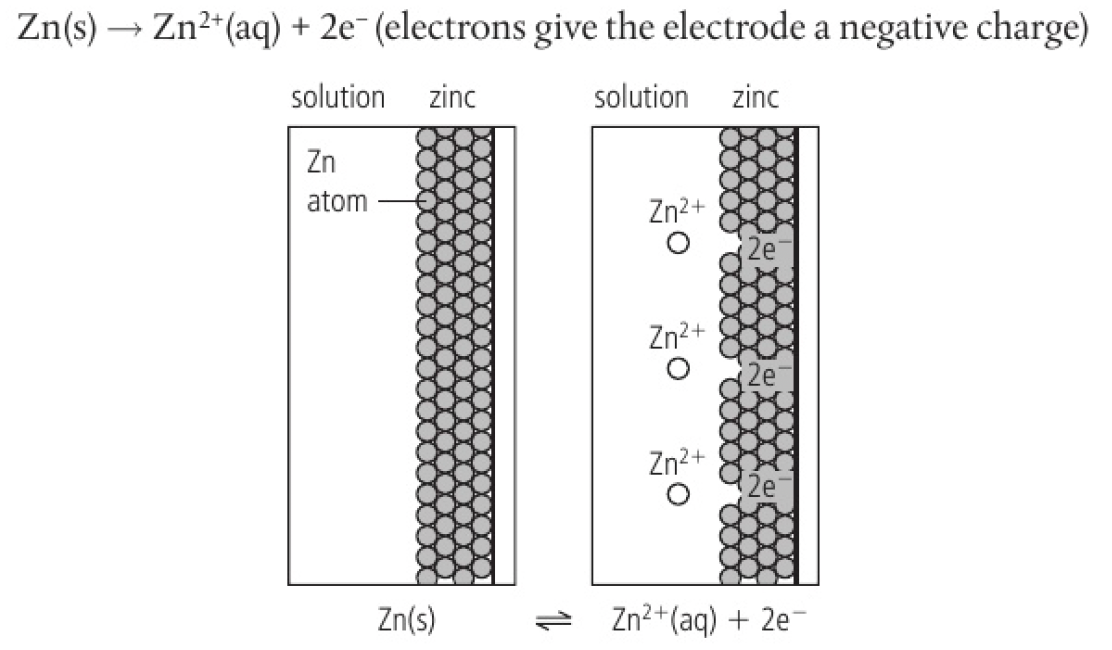
the electrons give the electrode a negative charge
the negative charge on the electrode attracts zinc ions which can gain electrons in the reverse process
at a particular charge separation, the rates of both processes will be the same and equilibrium is reached:
the position of this equilibrium depends on the reactivity of the metal:
- the more reactive, the more towards ions
- the less reactive, the more towards solid metal
as copper is a less reactive metal, the equilibrium position favours the formation of metal.
the copper metal strip will have fewer electrons and be less negative than the zinc metal strip.
when the two half-cells are connected by an external wire and a salt bridge, electrons will have a tendency to flow spontaneously through the external circuit from the more negative zinc half-cell to the less negative copper half-cell.
the tendency for electrons to flow can be quantified by measuring the potential difference between the two electrodes with a volt meter.
Important
oxidation always happens at the anode
reduction always happens at the cathode
think of an oil rig cat
the anode is negative as the reaction goes towards electrons
the cathode is positive as the reaction goes from electrons to neutral metal
external electronic circuit:
- required for electron flow
salt bridge:
- glass tube / strip of absorptive paper with aqueous ions
- movement of ions neutralises build up of charge and potential difference
- anions move from cathode to anode
- anions move in opposite direction to electrons
- cations move from anode to cathode
- solutions chosen should not interfere with reactions at electrodes
cell diagram convention
cell diagram
- | single vertical line represents a phase boundary
- || double vertical line represents salt bridge
- aqueous solutions of each electrode are placed next to the salt bridge
- anode on left, cathode on right
- electron flow is from left to right
- spectator ions omitted
- half-cells with two ions separate ions with comma (in same phase)
eg
the cell diagram is set out to show the direction of the two half reactions
different half-cells make voltaic cells with different voltages
the voltage generated between half-cells is determined by the difference in reducing strength of the two metals. this can be judged by the relative position of the metals in the activity series.