Structure 3.2.6 - structural isomers are molecules which have the same molecular formula but different constitutions (atom identities, connectivities, bond multiplicities)
- branched, straight-chain, position, functional group isomers
- primary, secondary, tertiary alcohols
- halogenoalkanes
- amines
structural isomers have the same molecular formula but different arrangements. they have unique physical and chemical properties
alkanes can have straight chain or branched structural isomers
pentane’s isomers
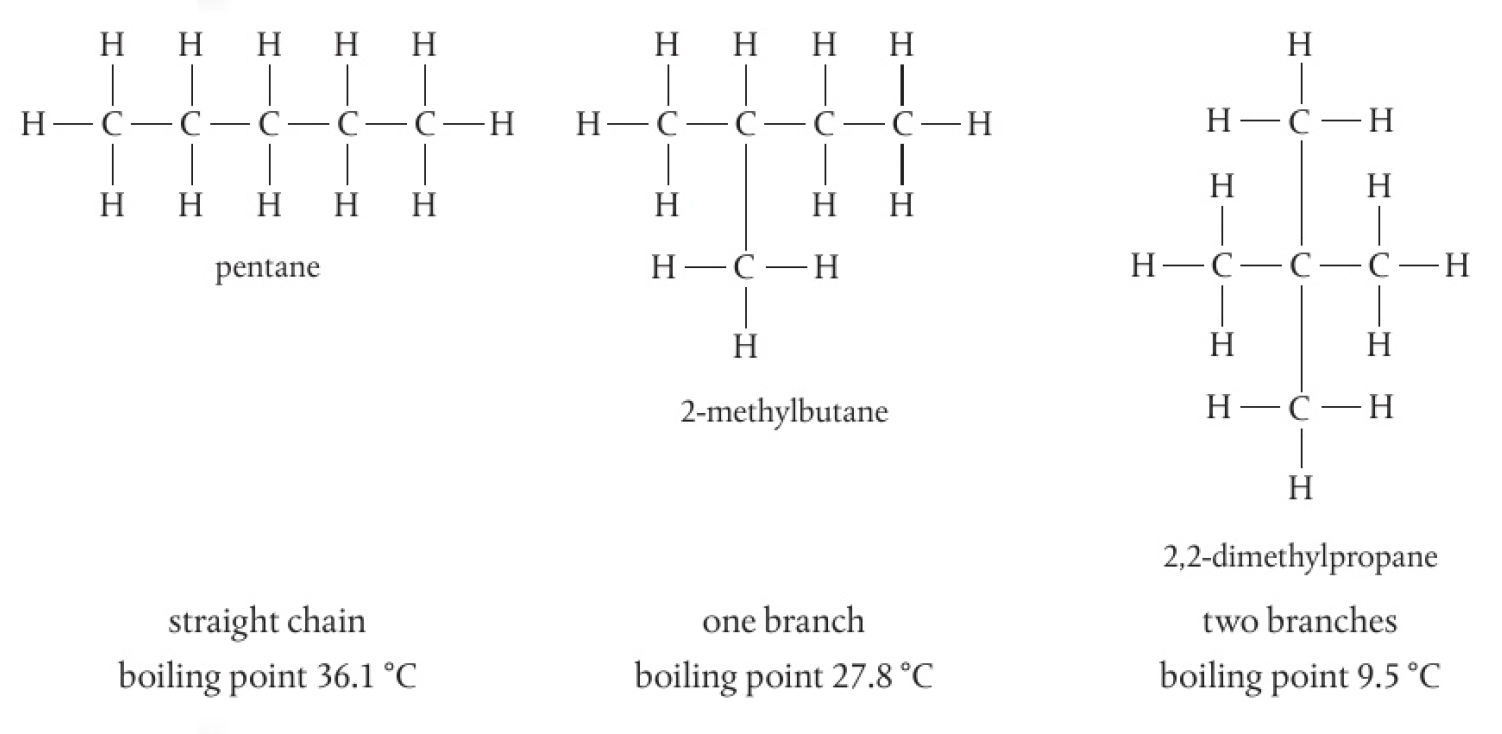
there is a trend in the boiling points of isomers: the more branching that is present in an isomer, the lower its boiling point
this is due to the reduction of amount of surface contact between neighbouring molecules, reducing the strength of the London dispersion forces
positional structural isomers
alkenes and alkynes can have positional structural isomers, where the double bond is found in different positions
structural isomers also occur in alcohols, halogenoalkanes and amines due to the position of their functional groups.
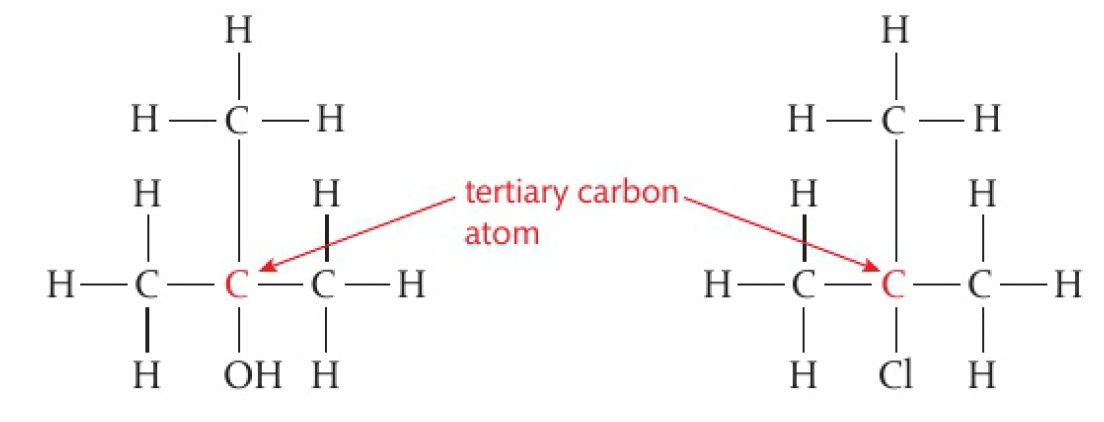
the primary carbon atom is attached to the functional group and also to at least two hydrogen atoms. these molecules are known as primary molecules

a secondary carbon atom is attached to the functional group and to 1 hydrogen atom and 2 alkyl groups. these molecules are known as secondary molecules.

a tertiary carbon atom is attached to the functional group and also bonded to 3 alkyl groups, 0 hydrogen atoms. these molecules are known as tertiary molecules
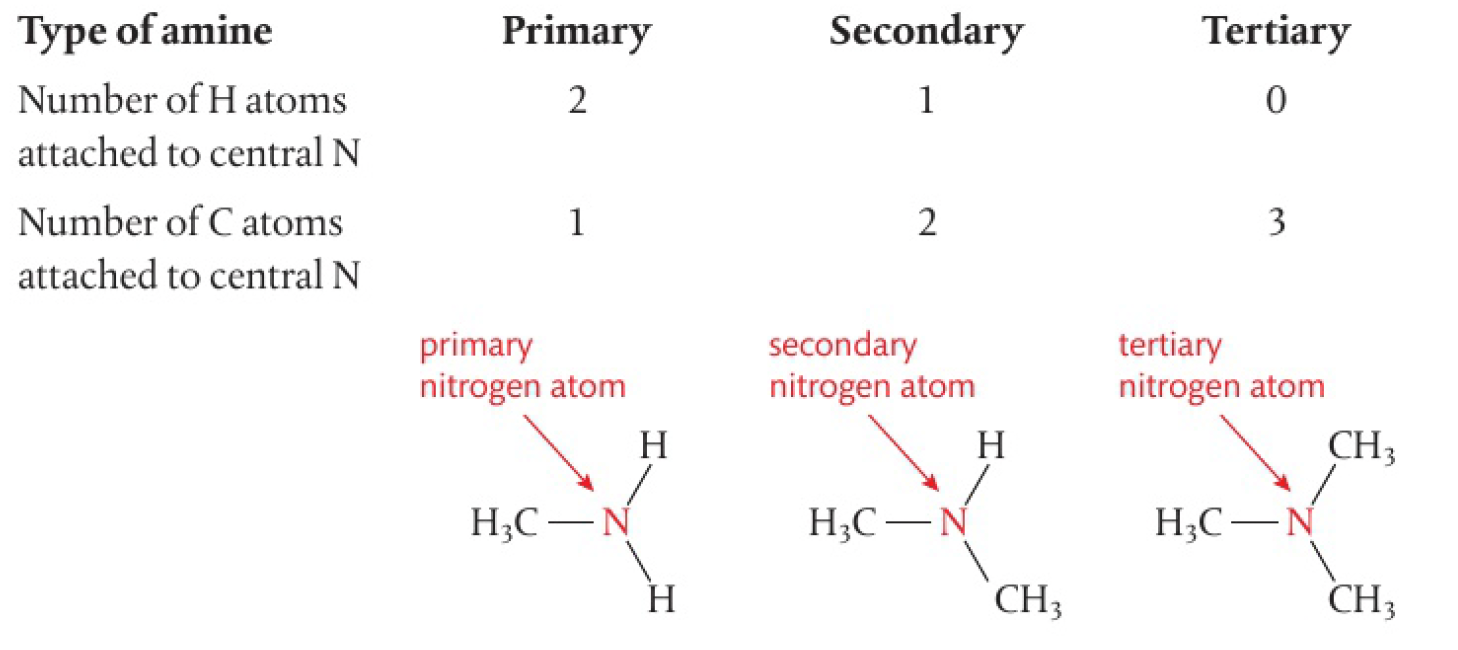
a similar classification can also be applied to amines according to the number of alkyl groups and hydrogen atoms bonded to the nitrogen atom.
functional group isomers
molecules can have the same molecular formula but different functional groups.
eg
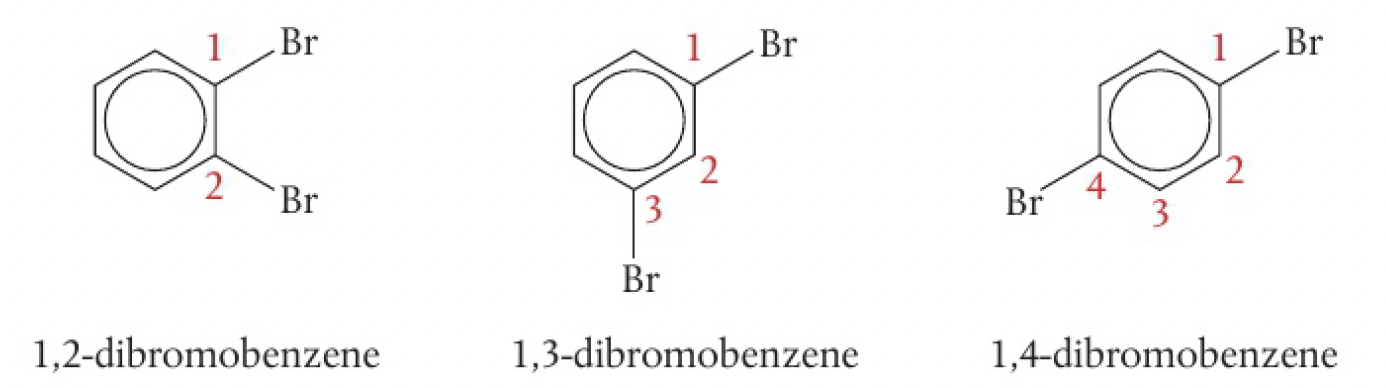
OCCCOCdisubstituted benzene
disubstituted benzene is when hydrogen atoms are replaced by halogen atoms or a functional group
for monosubstituted benzenes, there are no structural isomers since all six positions are identical, but 3 structural isomers are possible for disubstituted benzenes. this provides evidence for the accepted benzene model. see 2.2.12 benzene
challenge questions
- the subject guide only requires you to determine isomers for compounds containing up to six carbons in the longest chain. however, it can be beneficial to apply your knowledge to larger compounds. see if you can identify the nine structural isomers of heptane.
no thanks!
- dimethylbenzene (xylene) has 3 structural isomers. draw and name these isomers and predict which one would have the highest melting point
1,2-dimethylbenzene
c1c(c)c(c)ccc11,3-dimethylbenzene
c1c(c)cc(c)cc11,4-dimethylbenzene
c1c(c)ccc(c)c1all 3 isomers are non-polar, so the strongest intermolecular force would be London dispersion forces. to maximise strength, molecules should have a high degree of surface contact. 1,4-dimethylbenzene has the achieves the greatest surface contact out of the 3 isomers, so it has the highest melting point.