chem whatarethemechanismsofchemicalchange
Reactivity 3.2.12 - the hydrogen half-cell
a voltaic cell generates a potential difference known as the electromotive force (emf)
-
electrons generally tend to flow from the half-cell with the more negative potential to the half-cell with more positive potential
-
cell potential or electrode potential or
is the potential generated - measured with a voltmeter
- depends on the difference in the tendencies to undergo reduction
-
standard hydrogen electrode (SHE) is used as the baseline for measuring and comparing electrode potentials
-
also called the standard hydrogen half-cell
-
arbitrarily assigned an electrode potential of
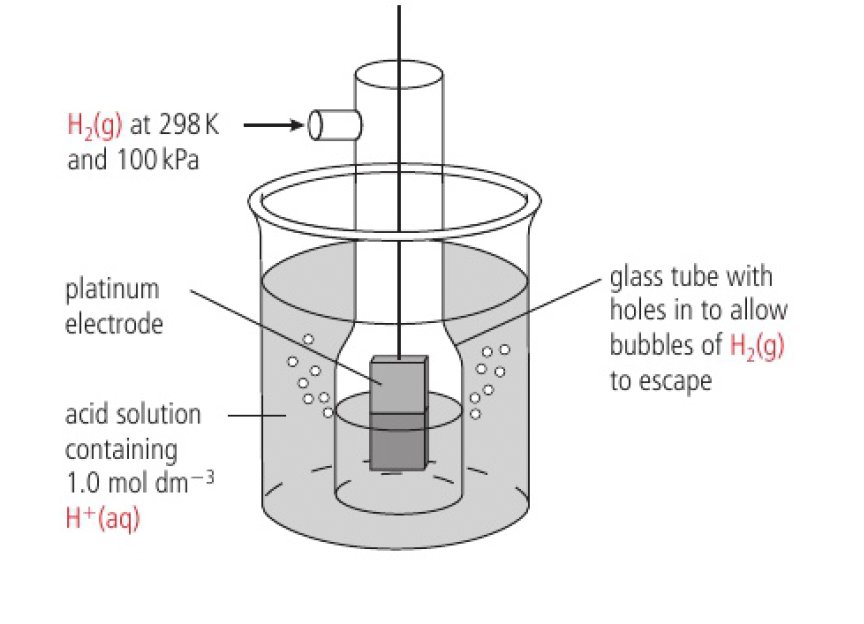
platinum is used as:
-
it is inert, will not ionise
-
catalyses the reduction of
ions -
the surface is coated with very finely divided platinum to increase the surface area
-
this increases adsorption of hydrogen gas
-
this increases the rate of reaction of both the forward and backward reactions
as the electrode is immersed in the acidic solution, an equilibrium is set up between the adsorbed layer of
measuring standard electrode potentials
standard conditions
- all solutions at concentration of
- gases at pressure of
- all substances pure
- temperature
- if the half-cell does not include a solid metal, platinum is used as the electrode
half-cells under these conditions are known as standard half-cells
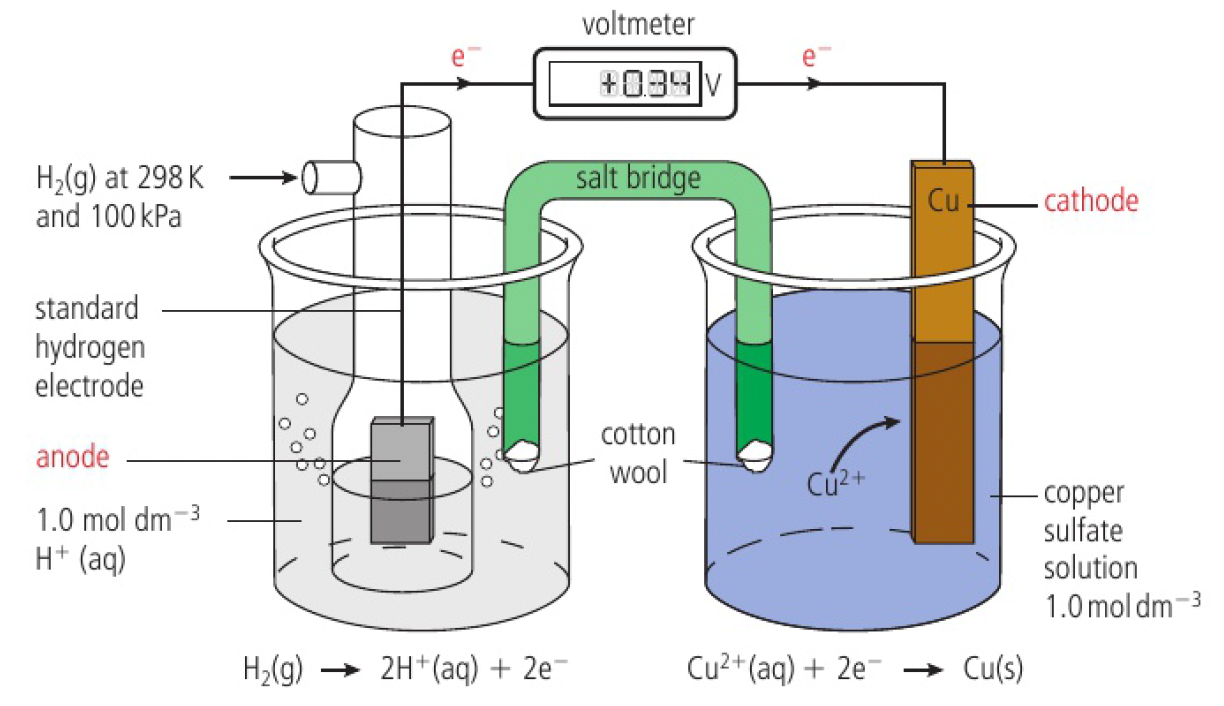
- emf generated,
- positive value shows that electrons tend to flow from the hydrogen to the copper half-cell
this indicates that electrons are being produced by the oxidation of
overall reaction:
this shows that
this can be represented with the cell diagram convention
standard electrode potentials are given for the reduction reaction
-
the standard hydrogen electrode is always connected to the negative terminal of the voltmeter
-
do not depend on the total number of electrons -
the more positive the
, the more readily it is reduced -
electrons always flow through the external circuit in a voltaic cell from the half-cell with the more negative standard electrode potential to the half-cell with the more positive electrode potential
-
oxidation occurs at the half-cell with more negative electrode potential
-
reduction occurs at the half-cell more positive electrode potential