chem whatdriveschemicalreactions
Reactivity 1.3.5 - a fuel cell can be used to convert chemical energy from a fuel directly to electrical energy
the hydrogen fuel cell:
this is a redox reaction that involves the transfer of electrons from hydrogen to oxygen. it can be used to produce an electric current directly if the reactants are physically separated.
in a fuel cell, reactants are continuously supplied to different electrodes. the hydrogen-oxygen fuel cell operates with either an acidic or alkaline electrolyte.
the main difference between a fuel cell and a primary (voltaic) cell is that fuel cells do not run out. the fuel is supplied continuously as it is oxidised. the chemicals in a primary cell stops working when the redox reaction is complete
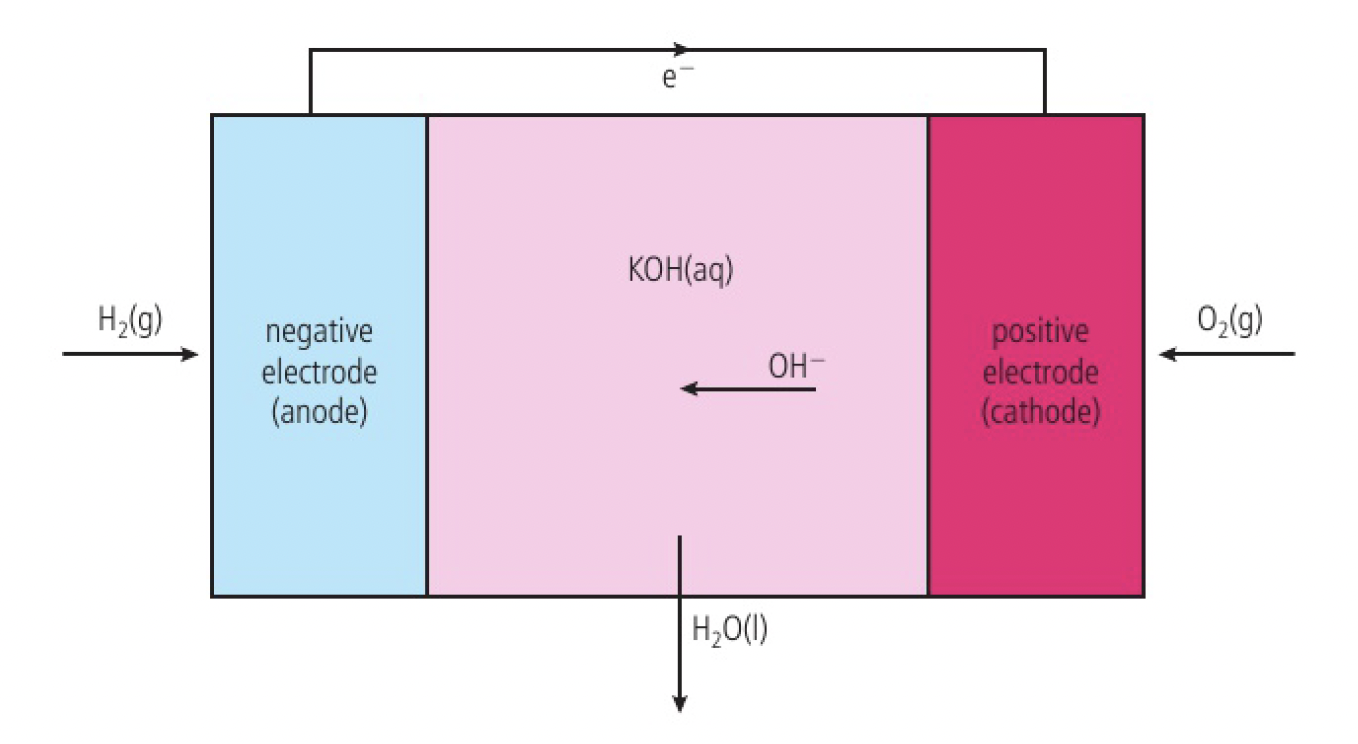
the fuel cell will function as long as hydrogen and oxygen are supplied. the electrodes are often made of porous carbon with added transition metals. the potassium hydroxide provides the hydroxide ions that are transferred across the cell.
one problem is that hydrogen gas is almost never found as the element in nature and needs to be extracted from other sources.
methanol
methanol is:
- stable liquid at normal environmental conditions
- has a high energy density
- easy to transport
in the direct methanol fuel cell, the fuel is oxidised under acidic conditions on a catalyst surface to form carbon dioxide. the
challenge questions
- explain the increased efficiency of a hydrogen-oxygen fuel cell which produces steam compared with a cell that produces water.
the entropy decrease is smaller for the reaction which produces one mole of gaseous water, which leads to a greater efficiency