chem modelsofbondingandstructure
Structure 2.2.16 - hybridisation is the concept of mixing atomic orbitals to form new hybrid orbitals for bonding
concept
carbon forms 4 covalent bonds, despite its two singly occupied orbitals available for bonding:
the fact that carbon forms 4 covalent bonds indicates that this ground-state electron configuration changes during bonding.
excitation is when an electron is promoted within the atom from the 2s orbital to the vacant 2p orbital
the atom now has 4 singly occupied orbitals available for bonding. hund’s rule states that all electrons in singly occupied orbitals in the same sub-shell have parallel spin, so:
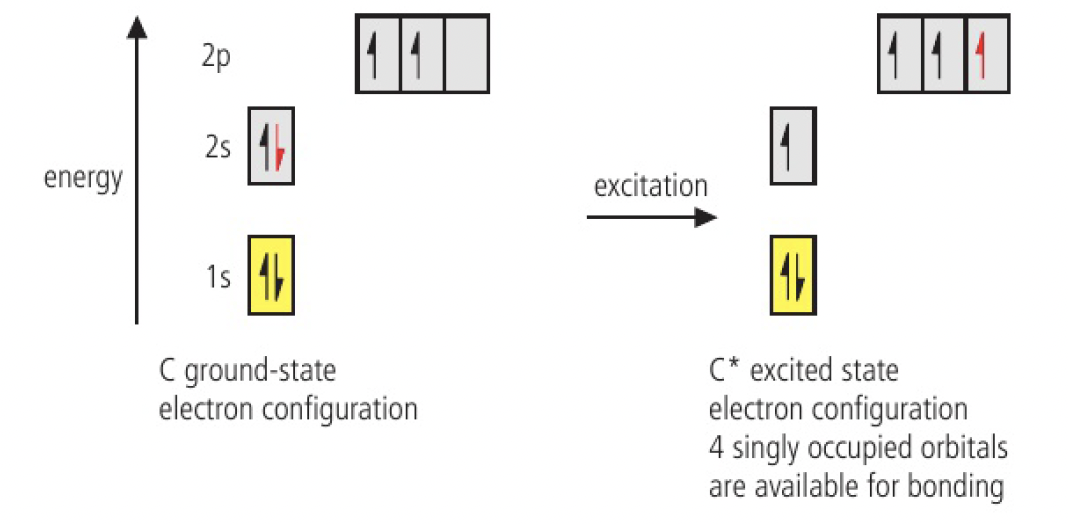
the amount of energy to excite the electron is compensated by the energy released on forming four bonds
the excited carbon has 1 singly occupied s orbital and 3 singly occupied p orbitals, which are at different energy levels. this means that if the orbitals were to participate in covalently bonding, the bonds would be unequal. however,
in essence, unequal atomic orbitals within an atom mix to form new hybrid atomic orbitals which are the same as each other, but different from the original.
hybrid orbitals have different energies, shapes, and orientation in space and are able to form stronger bonds by allowing for greater overlap.
orbitals are formed when 1 s orbital and 3 p orbitals are hybridised, producing 4 equal orbitals which are more p than s in character orbitals are formed when 1 s orbital and 2 p orbitals are hybridised, producing 3 equal orbitals orbitals are produced when 1 s orbital and 1 p orbital are hybridised, producing 2 equal orbitals
the different types of hybrid orbitals have different orientations.
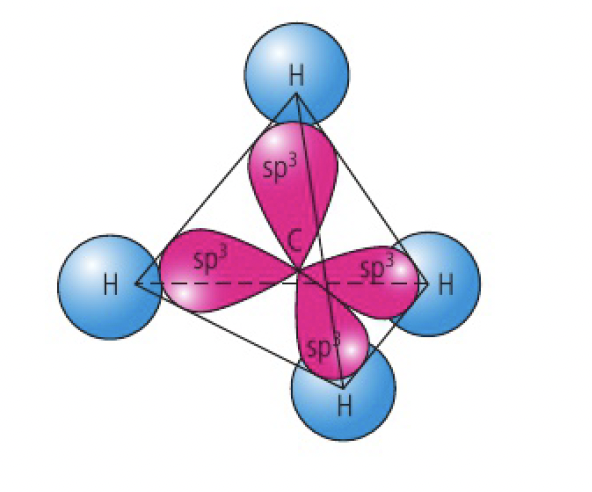
when carbon forms four single bonds, it undergoes
eg
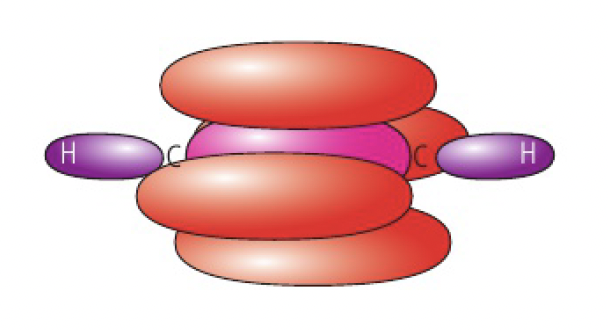
when carbon forms a double bond, it undergoes
eg
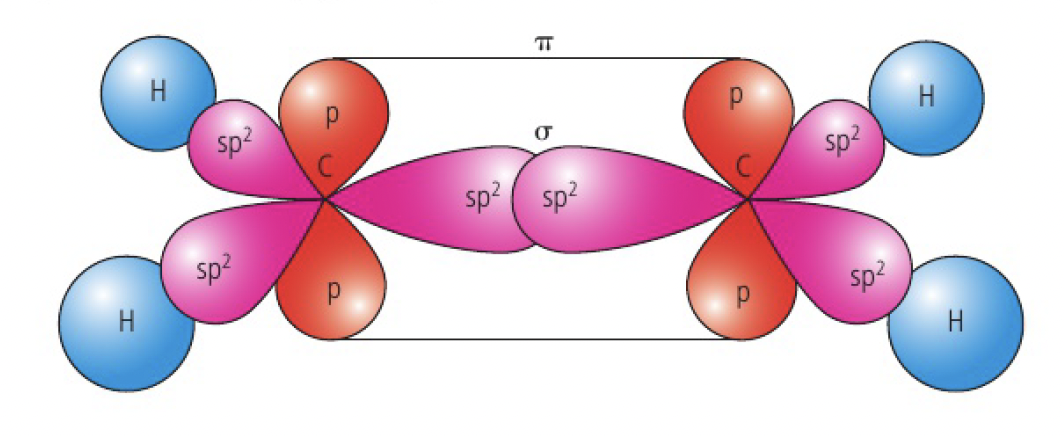
as the two carbon atoms approach each other, the p orbitals that have retained their dumbbell shape overlap sideways, forming a pi bond. thus, the double bond between carbon atoms consists of 1 sigma and 1 pi bond
when carbon forms a triple bond, it undergoes
eg
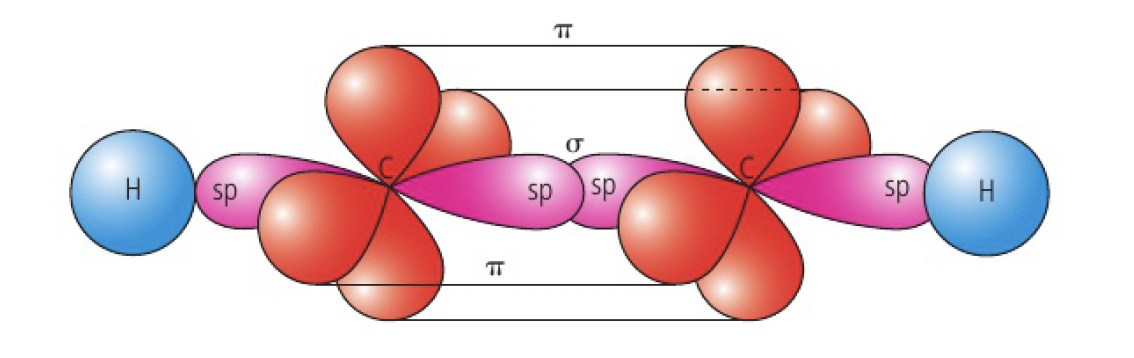
each carbon has two unhybridised p orbitals that are oriented at 90
lone pairs can also be involved in hybridisation. eg in
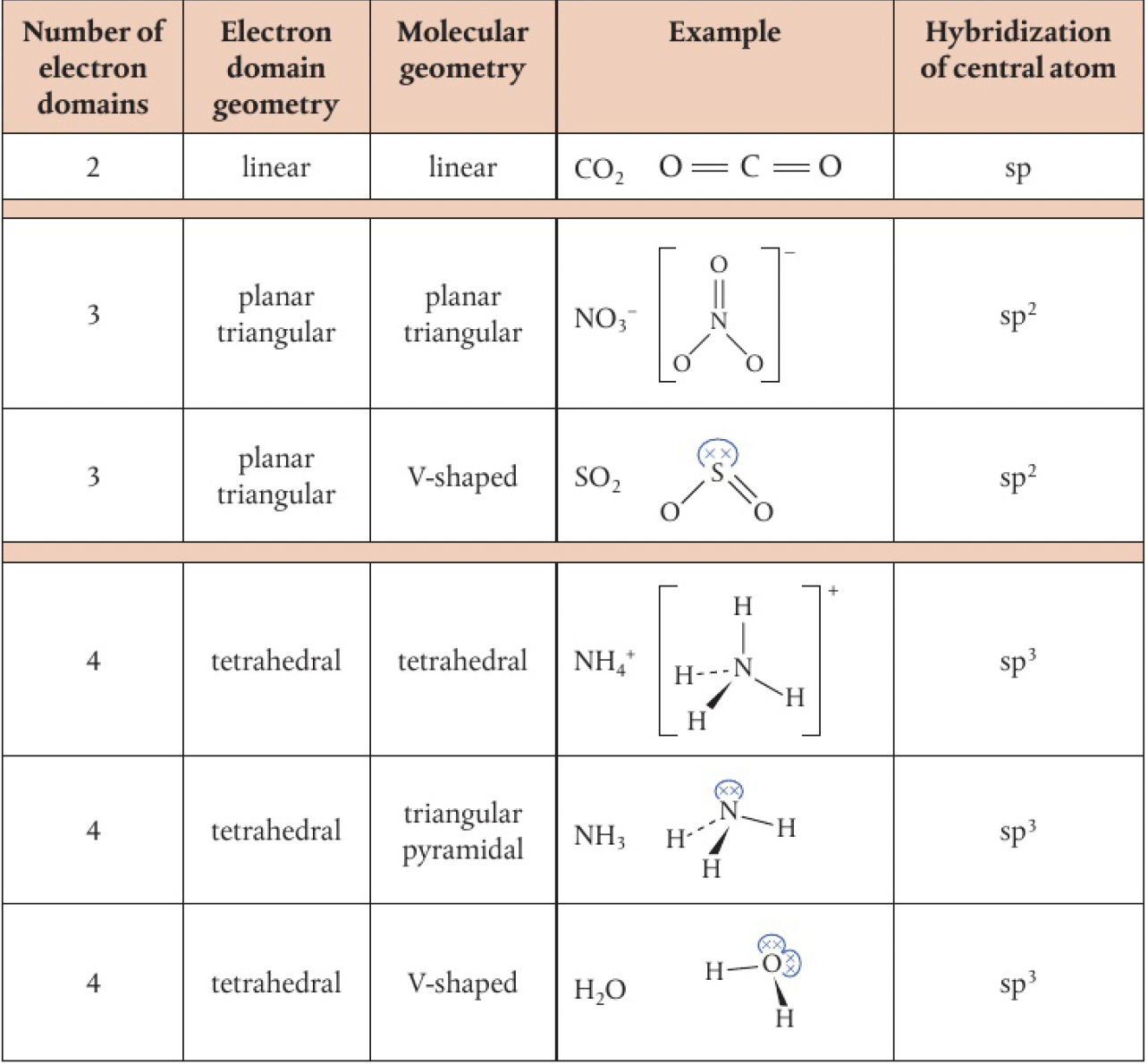
hybridisation and molecular geometry
hybridisation can also be used to predict molecular geometry
sp3d and sp3d2
- tetrahedral arrangement
hybridised - triangular planar arrangement
hybridised - linear arrangement
hybridised
the lone pair resides in one of the sp3 hybrid orbitals
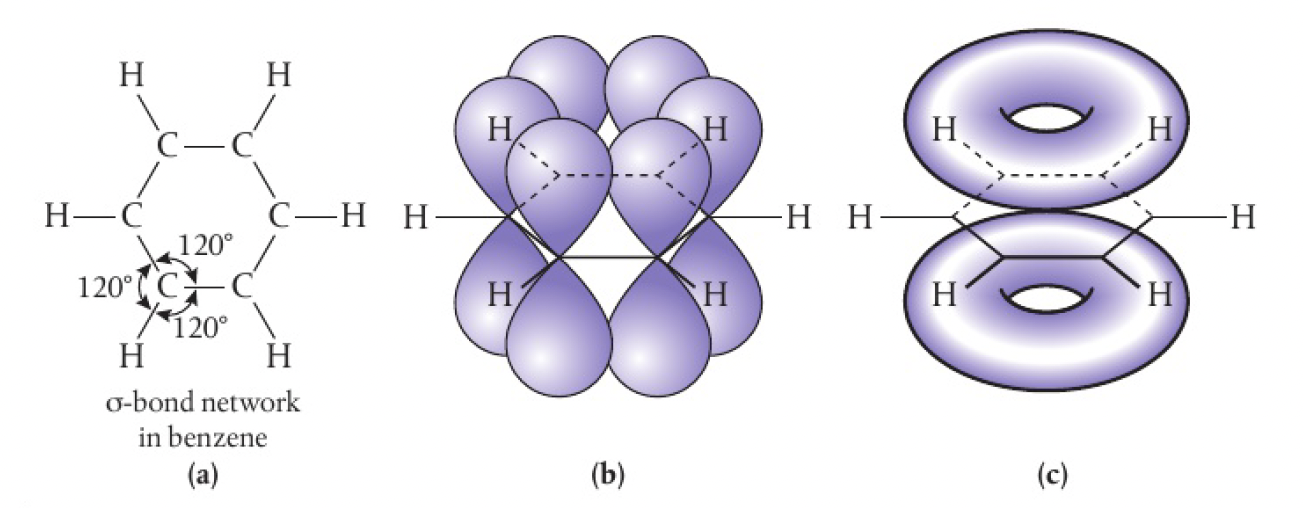
hybridisation in benzene
each of the six carbon atoms is
instead of the p orbitals pairing to form discrete alternating
challenge questions
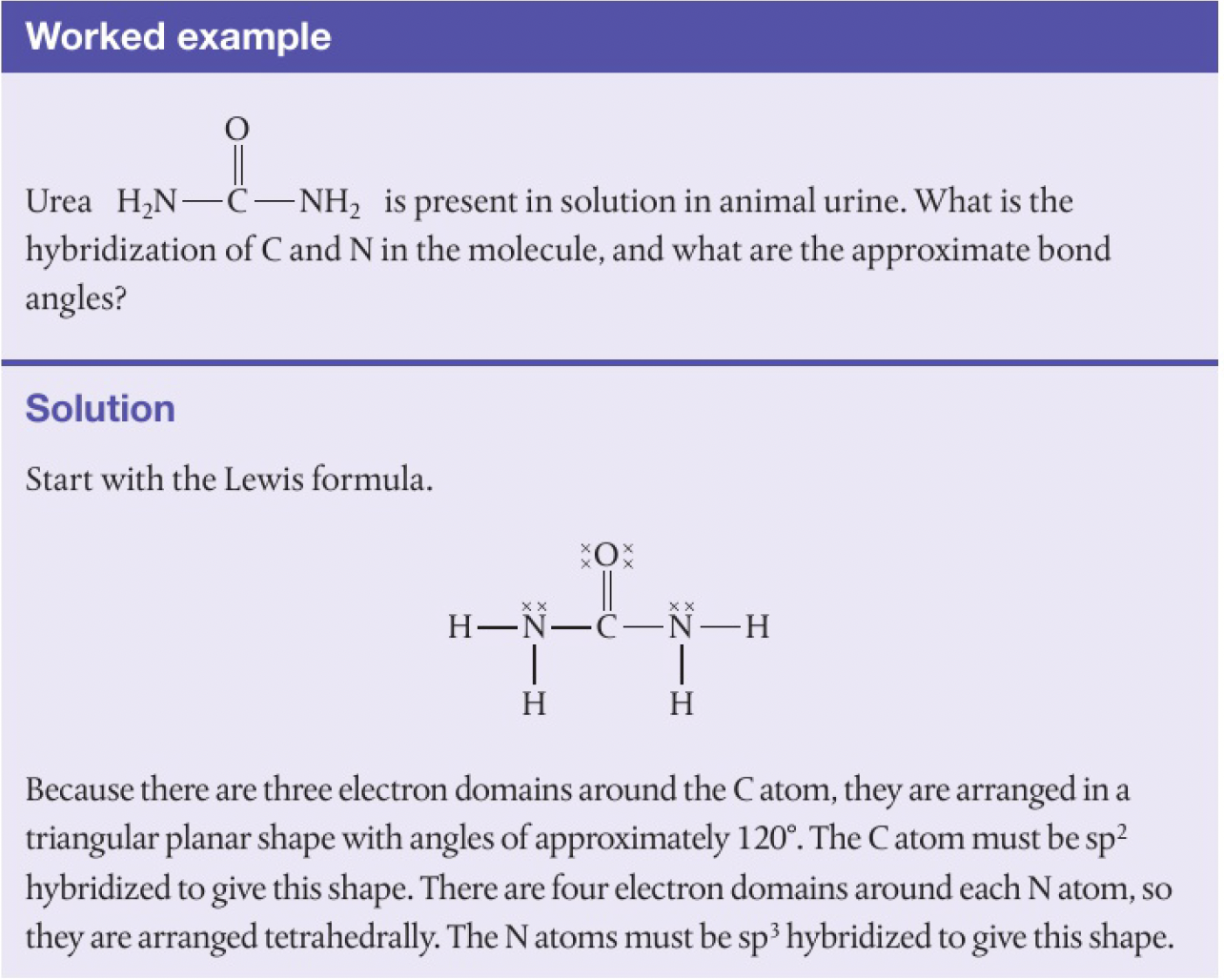
- molecules with expanded octets require more than 4 hybrid orbitals around the central atom. this involves the use of d orbitals in addition to s and p orbitals. what type of hybrid orbitals would be formed by the phosphorus atom in
, and the sulfur atom in ?