Reactivity 2.3.2 - the equilibrium law describes how the equilibrium constant,
Reactivity 2.3.3 - the magnitude of the equilibrium constant indicates the extent of a reaction at equilibrium and is temperature dependent
the equilibrium constant, K, has a fixed value for each reaction at a specified temperature.
for a balanced reaction:
the equilibrium constant expression is:
for the reverse reaction:
the equilibrium constant expression is:
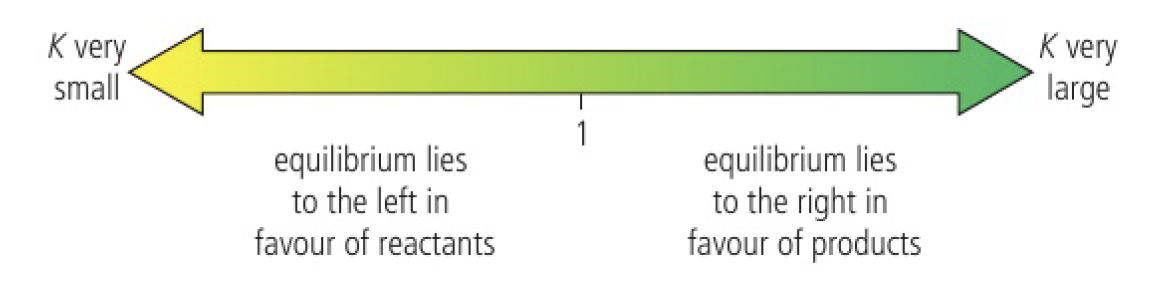
if:
, the reaction hardly proceeds equilibrium lies towards the reactants significant amounts of both reactants and products at equilibrium equilibrium lies towards the products reaction goes almost to completion
the magnitude of
the value of
challenge questions
- why do you think the reactions of the three halogens,
, , and , with have such different values for their equilibrium constant at the same temperature? what can you conclude about the strength of bonding in the three hydrogen halides?
the strength of bonding in the hydrogen halides increases as the electronegativity of the halide increases
- we can also express the relationship between
and for multiples of a reaction. doubling the reaction coefficients squares the equilibrium expression, , and halving the reaction coefficient square roots the equilibrium expression,
at a given temperature, the reaction:
has a value of
a)
b)
c)