chem modelsofbondingandstructure
Structure 2.2.11 - resonance structures occur when there is more than one possible position for a double bond in a molecule
in some molecules, bonding electrons are not confined to one location, but rather show a tendency to be shared between more than one bonding position - delocalisation
the delocalised electrons spread themselves out, giving greater stability to the molecule or ion.
e.g.: ozone,
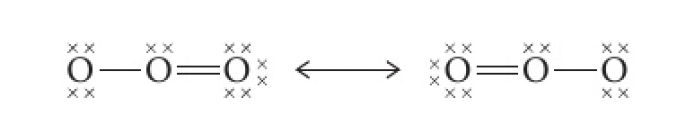
these structures suggest that the molecule should contain 1 double bond and 1 single bond, which would have differing bond strengths and lengths. however, experimental data shows that ozone contains two equal oxygen-oxygen bonds, intermediate in length and strength between single and double bonds.
thus, the true structure of ozone would be a combination, or a resonance hybrid of the two structures shown above. this is shown as
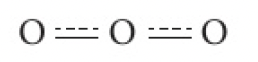
note: resonance structures do not represent forms that flip from one to the other.
also, resonance can be used to explain why
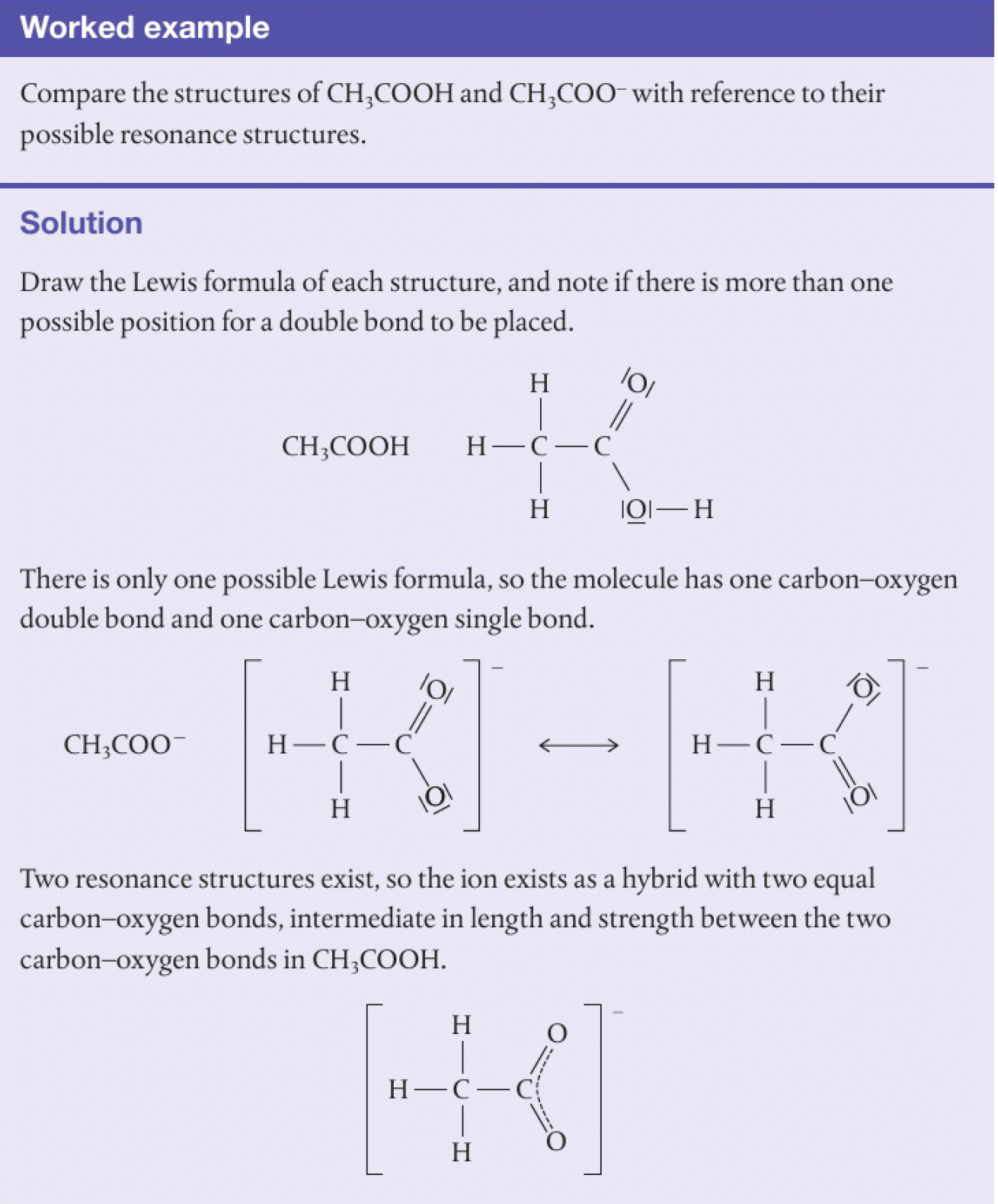
note: lone pairs of electrons are not shown on the structure of a resonance hybrid as they are involved in the process of delocalisation so do not have a fixed position.