Reactivity 2.2.1 - the rate of reaction is expressed as the change in concentration of a particular reactant/product per unit time
rate of reaction describes how quickly a reaction happens
NOTE
by convention, rate should be expressed as a positive value
the units are
the rate is the greatest at the start because that is when the reactant concentration is the highest. it is common to compare the initial rate by taking the tangent at
methods to measure rate of reaction
- in most cases, the concentration is not measured directly, but through different signals that are related to the changing concentration
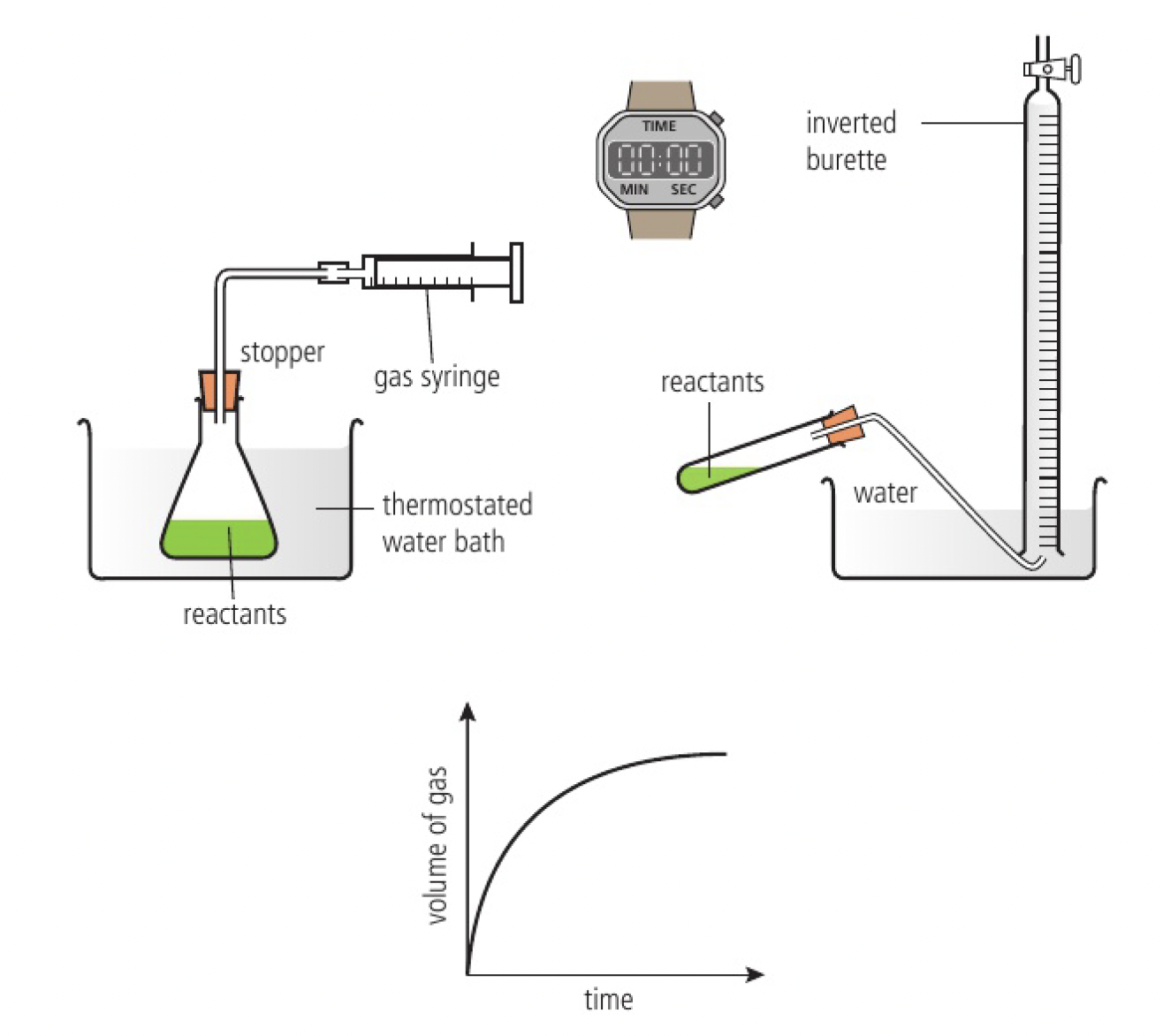
change in volume of gas produced
collecting the gas and measuring the change in volume at regular time intervals enables a graph to be plotted of volume against time
- gas syringe
- displacement of water from an inverted burette (only gases that have low solubility in water)
- data loggers for continuous monitoring
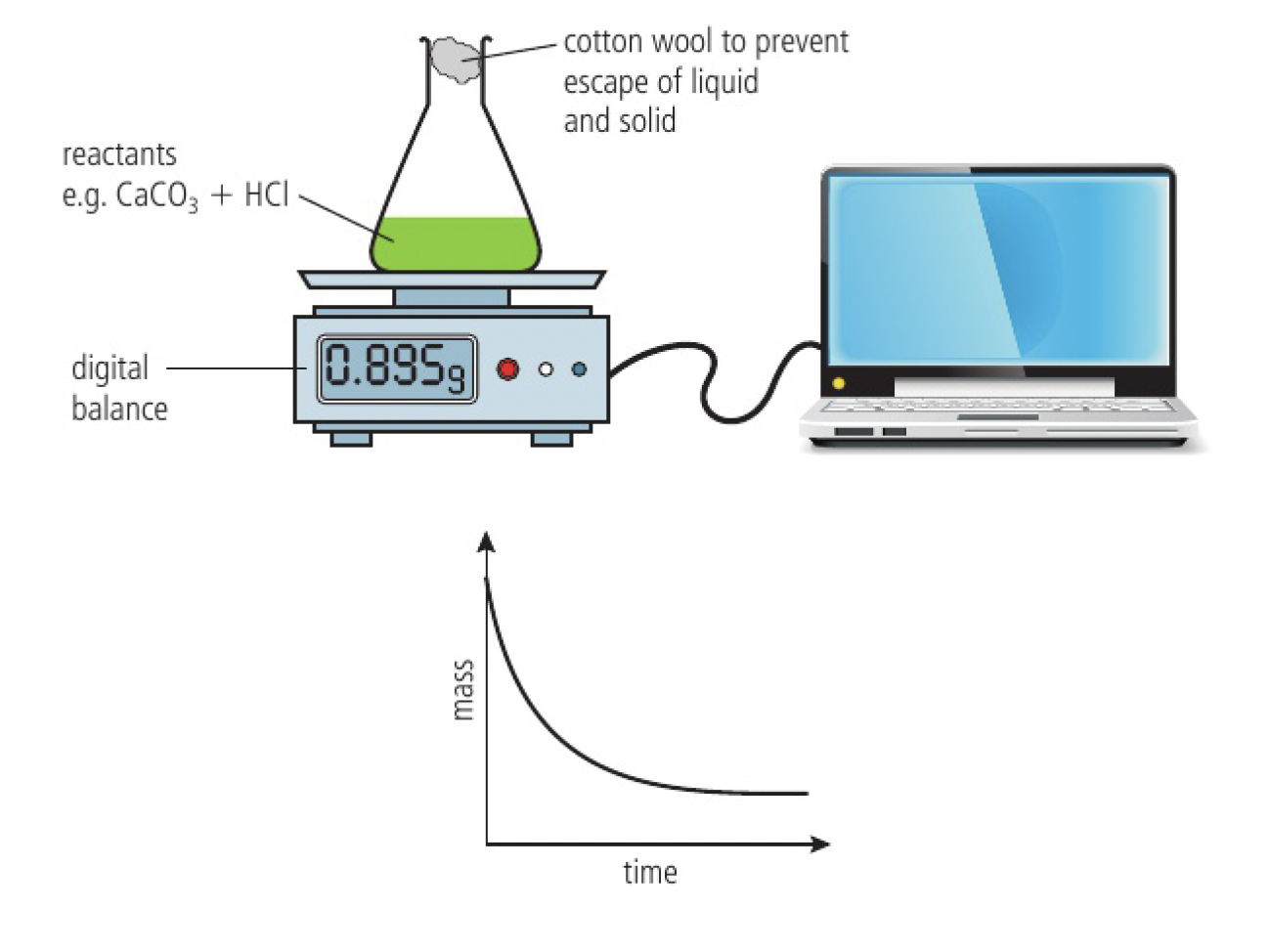
change in mass
it might be convenient to measure changes in mass with a balance. if the reaction is giving off a gas, the decrease in mass can be measured directly and continuously.
change in transmission of light: colorimetry/spectrophotometry
this technique can be used if one of the reactants or products is coloured. sometimes an indicator can be added to generate a coloured compound that can be followed. this allows for continuous readings to be made
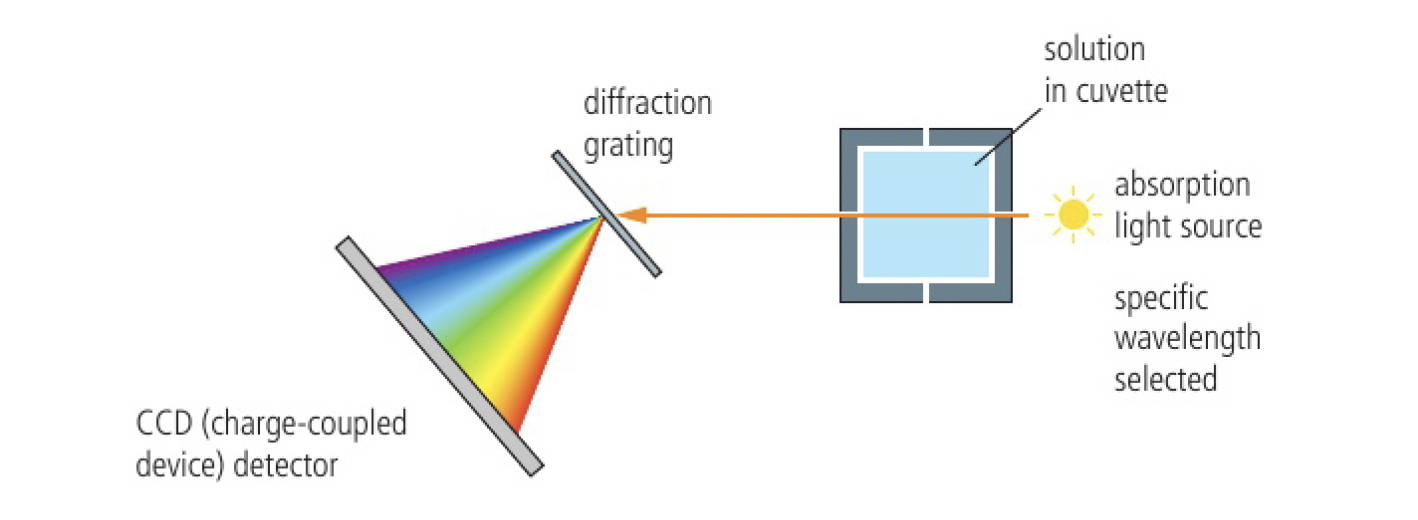
with a series of known solutions, the concentration can be found, however, it is often sufficient to record absorbance itself as a function against time
change in concentration using titration
samples are taken from the reaction mixture at regular time intervals and analysed by titration. as titration takes time, the reaction mixture in the sample will continue to react, so must be quenched. for example, adding a base to neutralise an acid.
(reaction between hydrogen peroxide and acidified potassium iodide)
iodine can be titrated against
change in conductivity
the total electrical conductivity of a solution depends on the total concentration of its ions and on their charges. conductivity can be measured directly using a conductivity meter. readings can also be converted into the concentrations of ions present with a series of known solutions.
for example:
non-continuous methods of detecting change during a reaction: ‘clock reactions’
sometimes it is difficult to record the continuous change in the rate of a reaction. then, the time it takes for a reaction to reach a certain chosen fixed point (something observable) is measured. the time taken to reach this point for the same reaction under different conditions can be compared and used as a means of judging the different rates of reaction.
here, time is used as a dependent variable.
the data obtained give only an average rate over the time interval.
- the time taken for a certain size piece of magnesium ribbon to react completely with dilute acid until it is no longer visible
- the time taken for a solution of sodium thiosulfate with dilute acid to become opaque by the precipitation of sulfur, so that a cross viewed through paper is no longer visible
challenge questions
- most gases are less soluble in warm water than in cold water, so using warm water may help to reduce a source of error when collecting a gas by displacement of water. but what new error might this create?
collecting a gas over warm water will increase its temperature and thus its volume.