Structure 3.2.7 - stereoisomers have the same constitution (atom identities, connectivities and bond multiplicities) but different spatial arrangements of atoms
stereoisomerism has molecules with atoms attached in the same order, but differing in spatial arrangement, requiring three-dimensional representation
graph TD A[Isomerism] B[Structural Isomerism] C[Stereoisomerism] D[Conformational Isomerism] E[Configurational Isomerism] F[Cis-Trans Isomerism] G[Optical Isomerism] A --> B A --> C C --> D C --> E E --> F E --> G
conformational isomers spontaneously interconvert through bond rotations, and so cannot be isolated separately. some conformers of a compound are more stable, so are favoured. (not required for ib)
configurational isomers have a permanent difference in their geometry. these cannot be interconverted and exist as separate compounds with distinct properties.
cis-trans isomers and alkenes and cyclic molecules
double-bonded molecules
the double bond consists of 1 sigma bond and 1 pi bond. free rotation around the double bond would not be possible as the
the reference plane is perpendicular to the sigma bonds and passes through the double bond.
when the molecule contains two or more different groups attached to the double bonded carbons, they can be arranged to give two isomers. the simplest, involving 2 different substituents, form cis and trans isomers. the prefixes are given in italics before the name
- cis refers to the isomer that has the same groups on the same side of the double bond or ring
- trans refers to the isomer that has the same groups on opposite sides
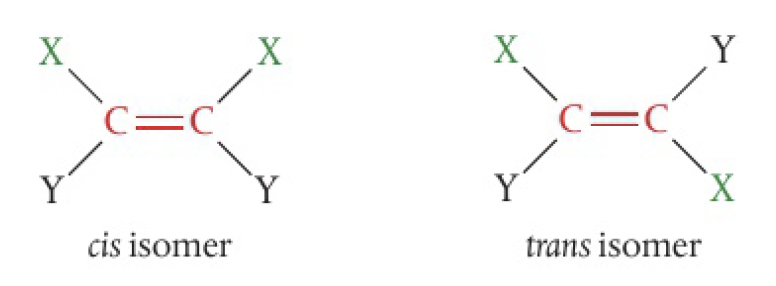
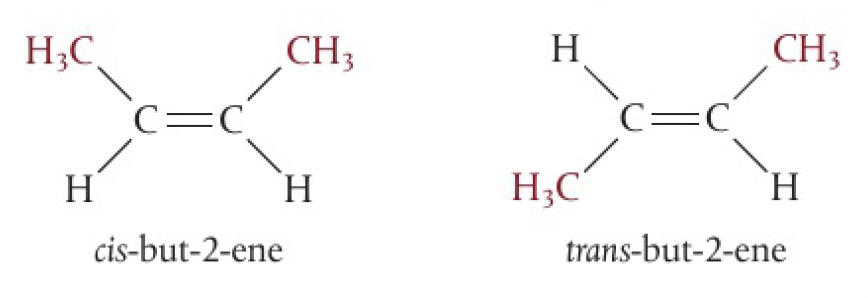
cyclic molecules
cycloalkanes contain a ring of carbon atoms that restricts rotation. the bond angles are strained from the tetrahedral angles in the parent alkane. in cyclopropane, the atoms form an equilateral triangle
c1cc1in cyclobutane, the atoms form a puckered square with approximate angles of 90
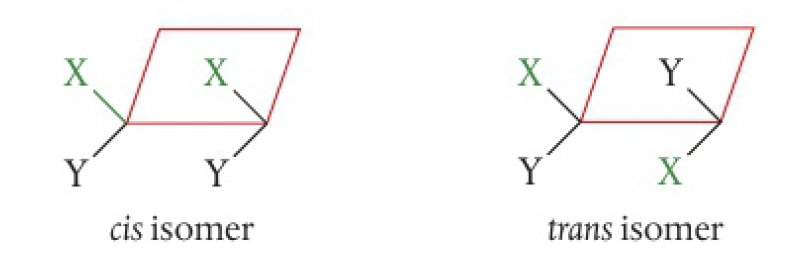
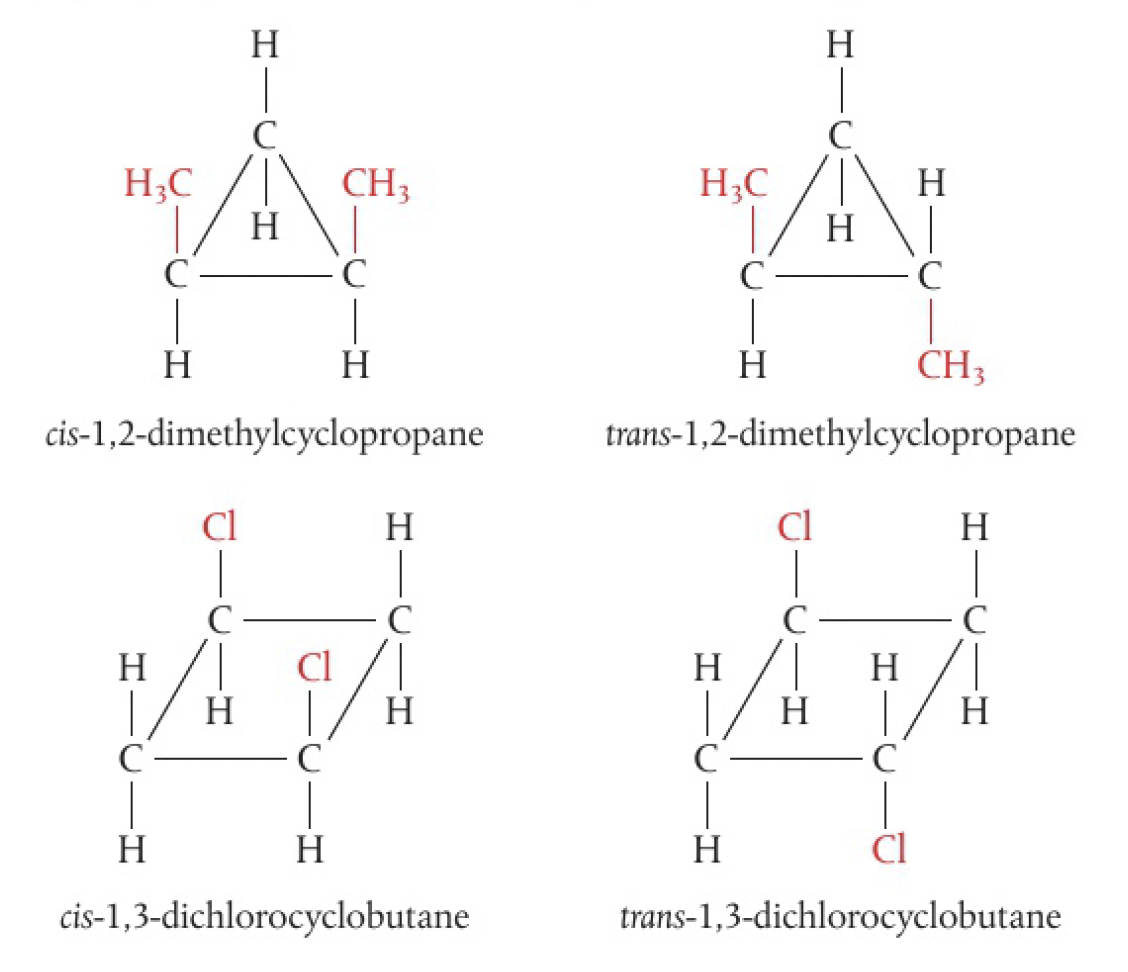
the position relative to the plane of the ring defines the isomer. note that the substituted groups do not have to be on adjacent carbon atoms.
1,3-dichlorocyclobutane and 1,2-dichlorocyclobutane would be positional structural isomers
optical isomers
a carbon atom attached to ==four different atoms or groups== is known as chiral, asymmetric, or a stereocentre. the four groups, arranged tetrahedrally around the carbon atom with bond angles of 109.5
enantiomers have opposite configurations at each chiral centre. some molecules have different configurations at one or more, but not all, chiral centres, known as diastereomers and are not mirror images of each other. eg. glucose and galactose. common sugars are often diastereomers of each other.
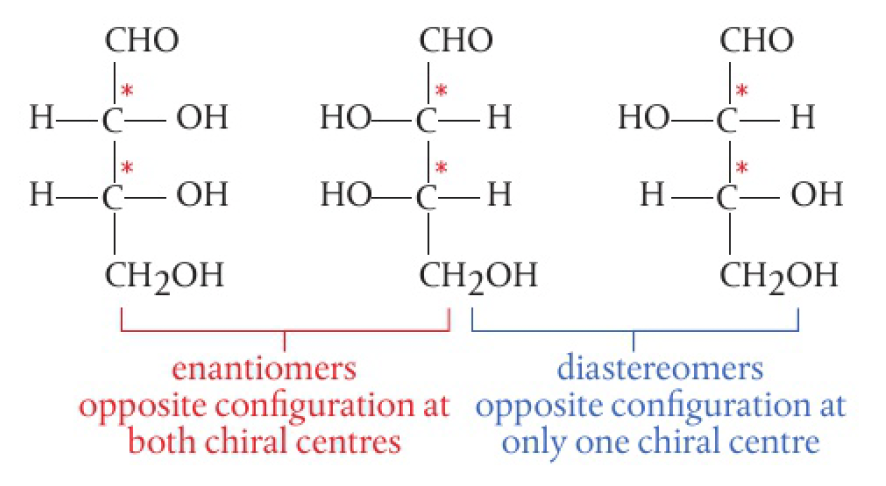
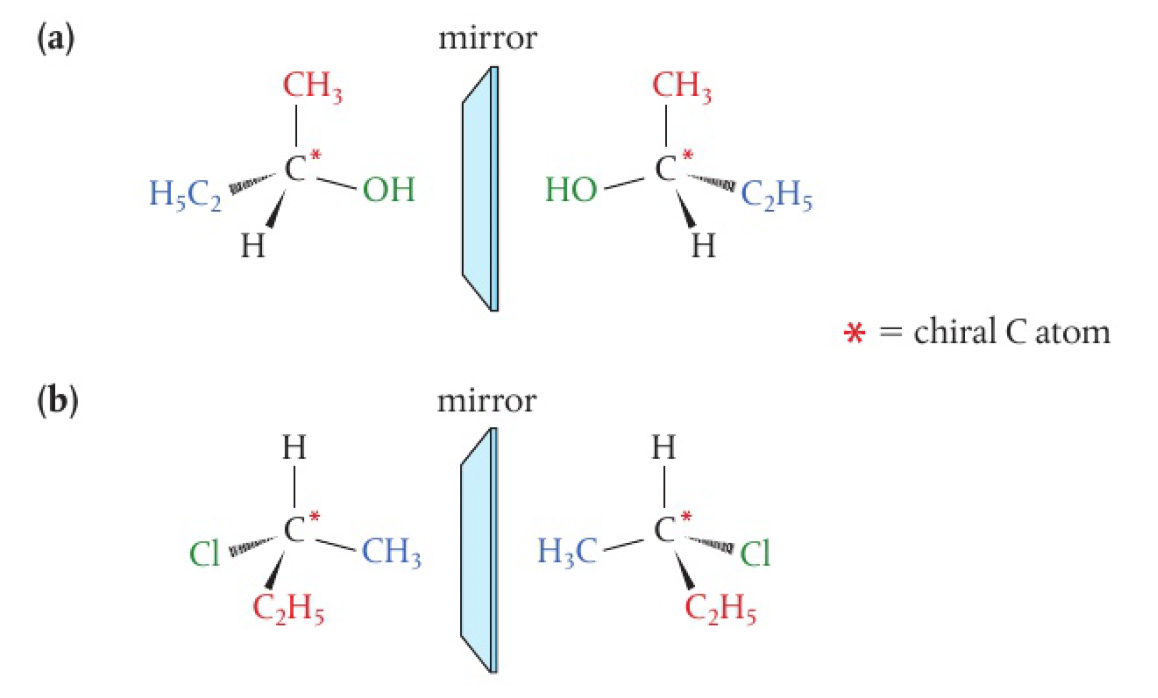
properties of enantiomers
- diastereomers usually differ in both physical and chemical properties.
enantiomers have identical physical and chemical properties with 2 exceptions
- optical activity
- reactivity with other chiral molecules
==optical activity==
optical isomers show a difference in a specific interaction with light
regular light consists of electromagnetic waves that oscillate in an infinite amount of planes at right angles to the direction of travel. passing this light through a polariser only allows light oscillating in 1 plane pass through. this is known as plane-polarised light.
a solution of optical isomers rotates plane-polarised light when a beam of it is passed through, which can be measured with a polarimeter
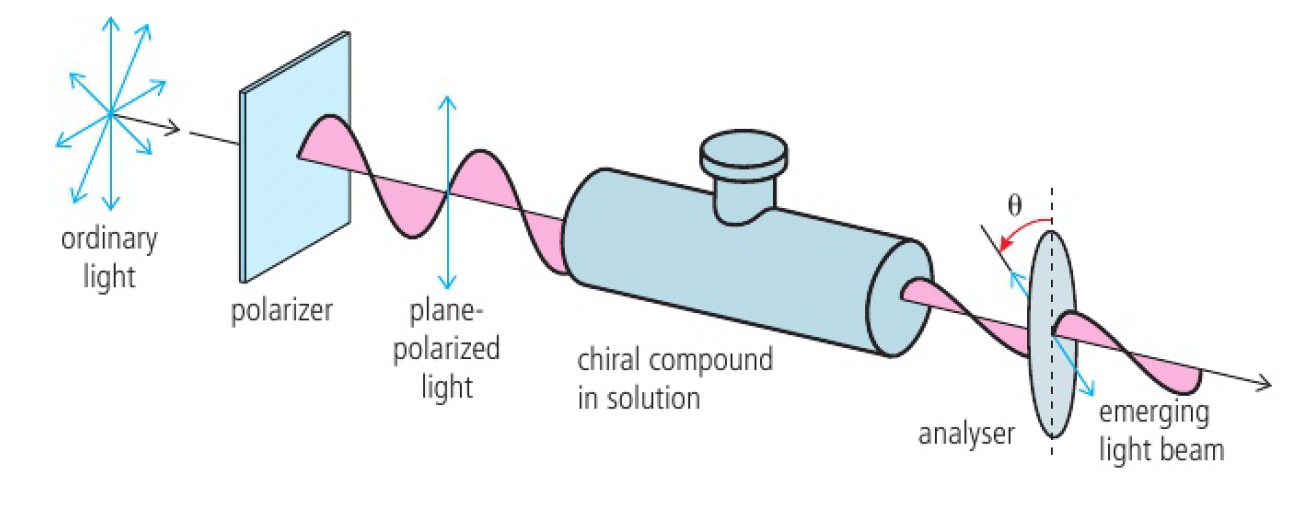
the analyser can be rotated until the light passes through it, so the angle of rotation (
separate solutions of enantiomers, at the same concentration, rotate plane-polarised light in equal amounts but in opposite directions, optically active.
a racemic mixture of a chiral compound contains equal concentrations of the two optical isomers. the rotation caused by each is cancelled out, so it is optically inactive.
- naturally occurring chiral molecules are optically active, and exist as only 1 enantiomer
- (-) enantiomers rotates light left
- (+) enantiomers rotates light right
reactivity with other chiral molecules
resolution is when a racemic mixture is mixed with a single enantiomer of another chiral compound, reacting to produce different products (because of the two enantiomers in the mixture) - with distinct chemical and physical properties - which can be separated.
asymmetric synthesis is the process for the manufacture of a single enantiomer using a chiral catalyst.
biological systems are chiral environments - thalidomide
challenge questions
- cis-trans isomerism can occur in inorganic as well as in organic compounds. think about why it can occur in square planar or octahedral complexes but not in tetrahedral complexes.
in both square planar and octahedral complexes, groups can be adjacent or across from each other, but in tetrahedral complexes, all groups are adjacent to each other.
- cis-butenedioic acid forms intramolecular hydrogen bonds at the expense of intermolecular bonds. consider what impact this may have on the physical properties and acid strength of the two isomers.
C(=O)(O)/C=C\C(=O)(O)the melting/boiling point of cis-butenedioic acid will be much lower than trans-butenedioic acid as it forms less intermolecular bonds
the solubility of cis-butenedioic acid is higher as it is a polar molecule (in trans-butenedioic acid, there is not a net dipole moment since it is symmetrical and is thus cancelled).
C(=O)(O)\C=C\C(=O)(O)when the
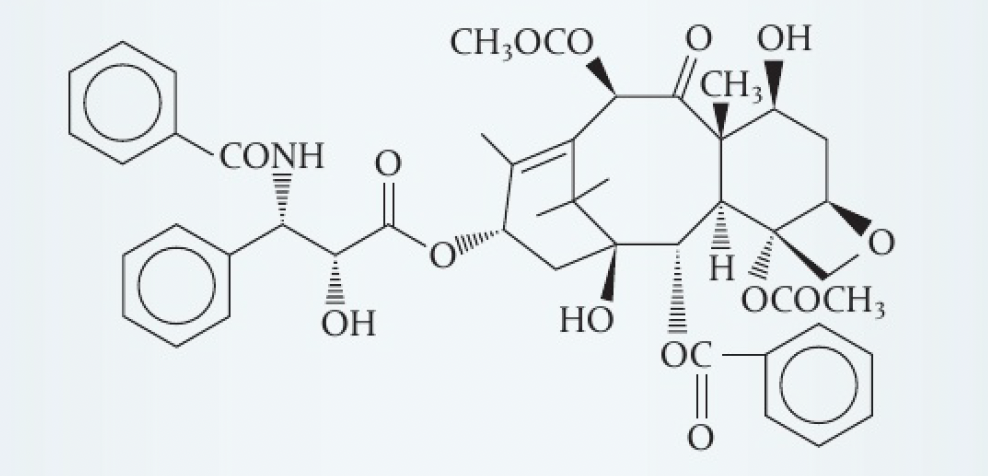
- the structure of taxol, an anti-cancer drug, is given below. taxol contains 11 chiral carbons. can you find them all?