chem whatarethemechanismsofchemicalchange
Reactivity 3.4.8 - coordination bonds are formed when ligands donate an electron pair to transition element cations, forming complex ions
see 3.1.10 coloured complexes (HL) and 3.1.6 oxidation states and 3.1.9 variable oxidation states (HL) and 3.1.8 characteristic properties of transition elements (HL)
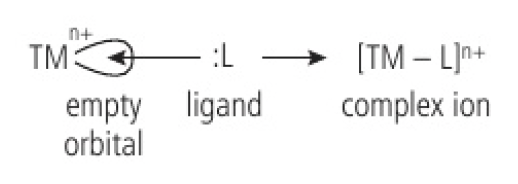
transition metal ions act as Lewis acids and ligands as Lewis bases
- transition metals in the middle of the periodic table often form ions with vacant orbitals in their
subshell - they act as Lewis acids and accept lone pairs of electrons when they bond with ligands to form complex ions
since the ligand donates both electrons, a coordination bond is formed
the chemical formulas for complex ions are always written inside square brackets with the charge given at the top right
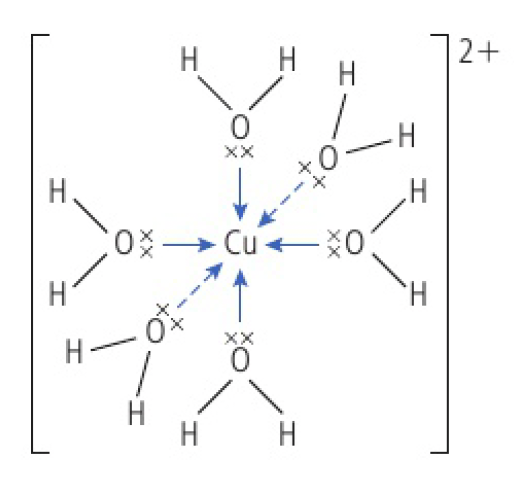
here,
ligands can be neutral molecules or anions but they must always contain at least one lone pair of electrons.
- neutral:
- anions:
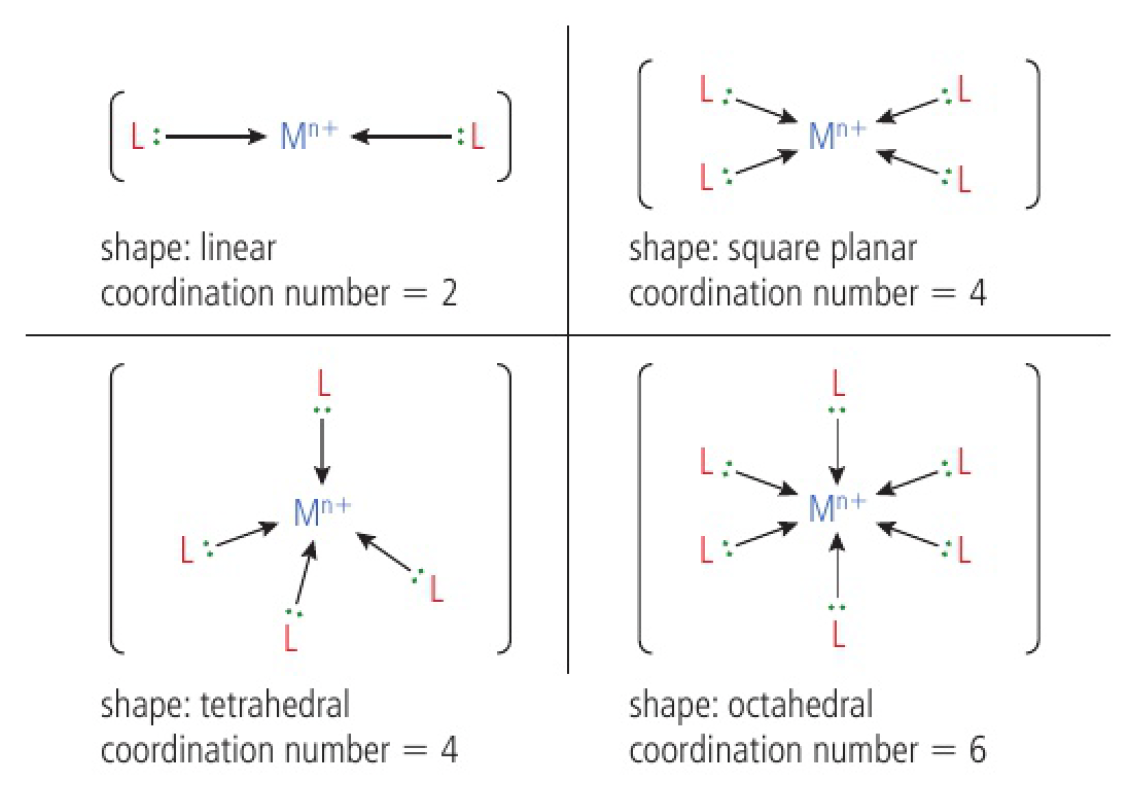
the charge on a complex ion depends on:
- the charge on the central transition metal ion
- the charge on the ligands
- the coordination number
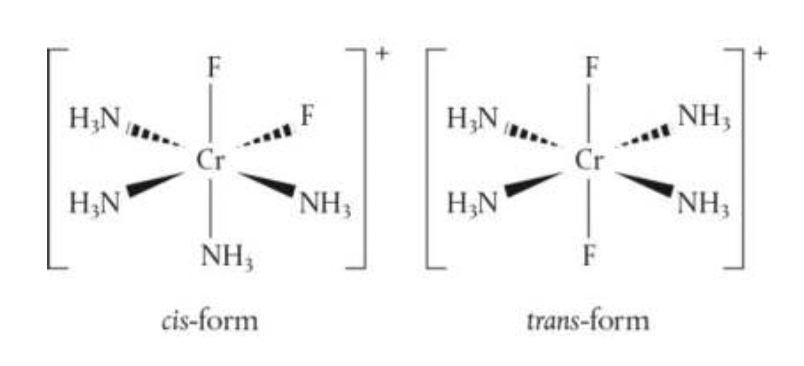
challenge questions
- complex ions can have stereoisomers, similar to those encountered for organic compounds. the complex ion
, included in the table, can exist as cis- and trans- isomers. see if you can deduce the difference between these two stereoisomers by drawing possible 3D structures of