Structure 3.2.10 - proton nuclear magnetic resonance (
Structure 3.2.11 - individual signals can be split into clusters of peaks
singlets, doublets, triplets, quartets
reading
NMR spectroscopy depends on the nuclear spin of atoms with an odd number of nucleons, which act as tiny bar magnets. when placed in an external magnetic field, the nuclei exist in 2 distinct energy levels depending on whether their magnetic field is aligned with or opposed to the external magnetic field. the energy gap between the energy levels is very small and only requires the absorption of low energy radio waves for the nuclei to move from the lower energy state to the higher energy state.
since electrons shield the nucleus from the full effect of the external magnetic field, differences in electron distribution produce different energy separations between the two spin energy levels. nuclei in different chemical environments produce different signals
H NMR gives information about the position of hydrogen atoms or protons in a molecule
MRI is an application of NMR as hydrogen atoms have a magnetic moment due to the odd number of nucleons, and hydrogen atoms are present in water, which makes up about 70% of the body by mass
each
- the number of signals in the spectrum
hydrogen nuclei that have the same chemical environment are said to be equivalent, and give the same signal in theNMR spectrum. the number of signals observed depends on the number of different chemical environments.
equivalent hydrogens can be identified by looking at the symmetry of the structure.
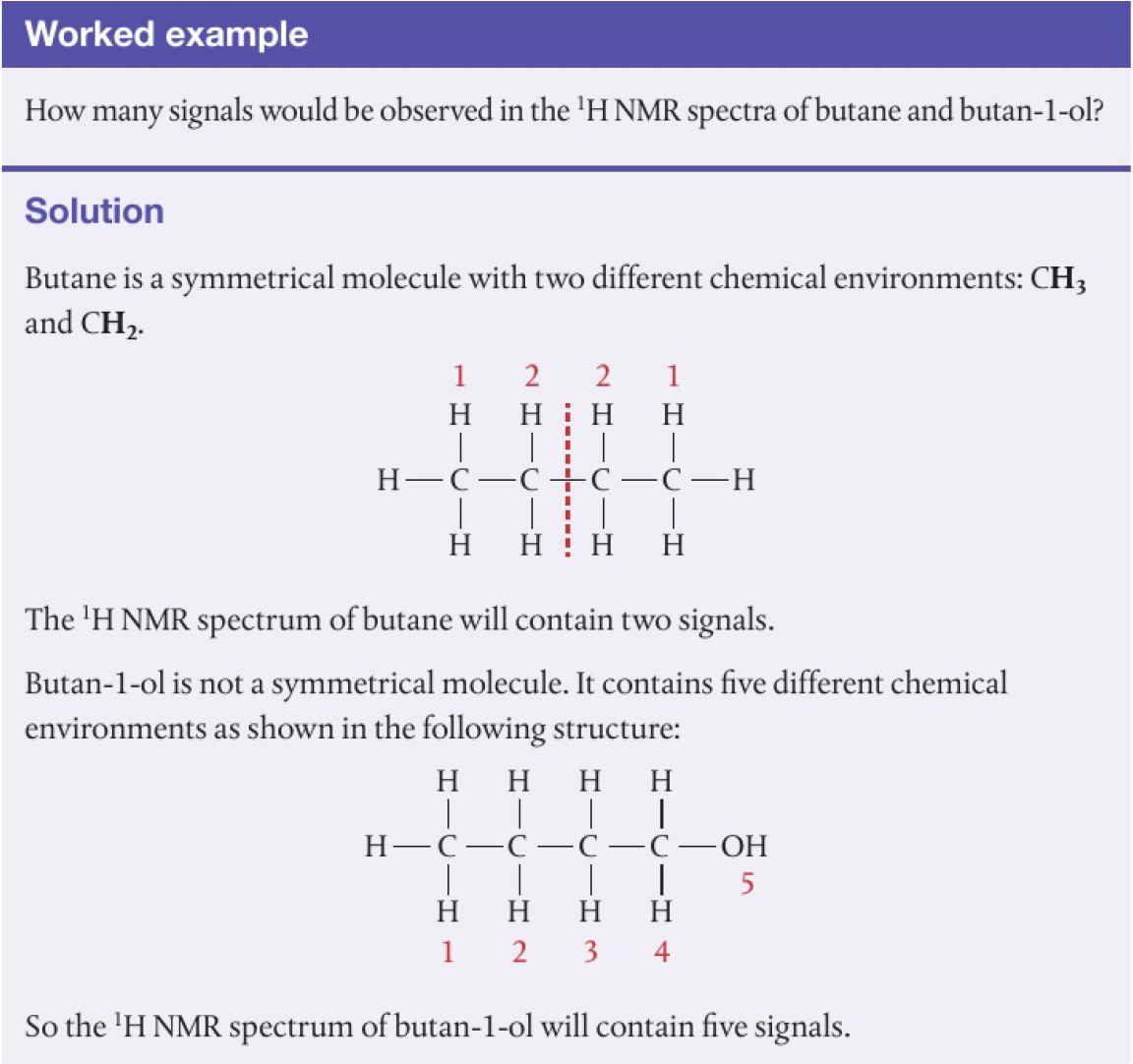
-
the position or chemical shift of each signal
chemical shift is the position of the x-axis. in an alcohol, the closer the hydrogen atoms are to the OH group, the higher the chemical shift observed. this happens because the high electronegativity of the oxygen atom pulls electrons away from the hydrogen atoms, causing the nuclei to be deshielded, making the nuclei more susceptible to the effects of the external magnetic field, and their signals occurring at a higher chemical shift. -
the size or integrated area of each signal
the area under a signal depends on the number of hydrogen atoms in the chemical environment.
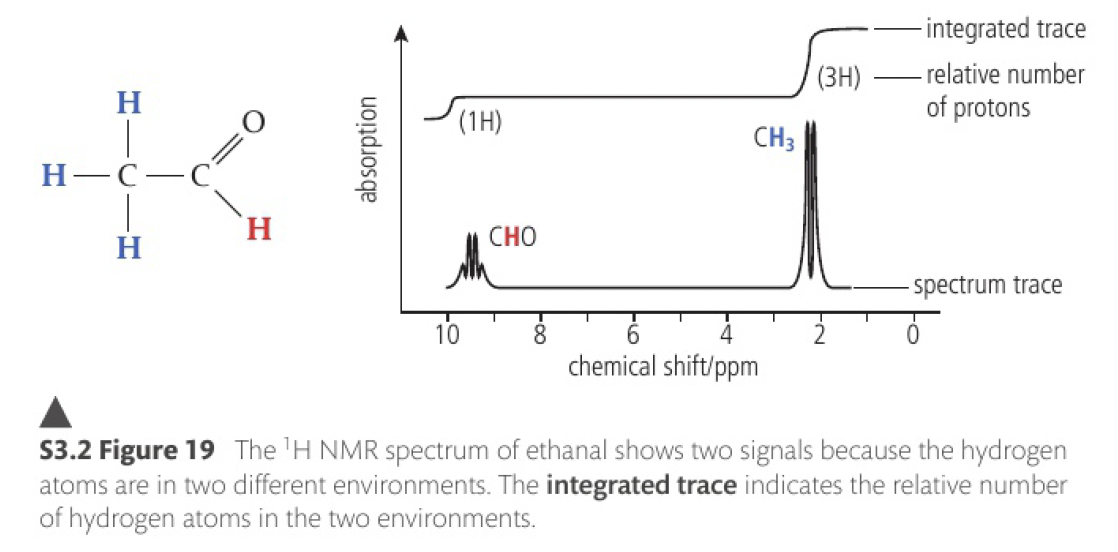
the relative intensity relates to its integrated area and is proportional to the number of hydrogen atoms in that chemical environment
- the splitting pattern observed for each signal
splitting occurs as the effective magnetic field is modified by the magnetic field produced by neighbouring protons.
this is known as spin-spin coupling
if there are n hydrogens on the neighbouring carbons, there will be n + 1 lines observed in the splitting pattern
the intensity of lines follows Pascal’s triangle
- protons bonded to the same atom do not interact with one another since they are equivalent and behave as a group
- protons on carbon atoms not adjacent to each other do not generally interact as they are too far apart for their magnetic fields to interact through spin-spin coupling
- alcohol protons typically do not engage in spin-spin coupling, so appear as singlets and do not split the signals of other protons