chem modelsofbondingandstructure
Structure 2.2.5 - bond polarity results from the difference in electronegativities of the bonded atoms
bonds are polar because of the difference in the electronegativities of bonded atoms, where the more electronegative atom exerts a greater pulling power on the shared electrons, gaining more possession, resulting in an unsymmetrical electron distribution.
bond dipole describes the partially separated opposite electric charges in polar bonds, with
pure covalent bonds are the truly non-polar bonds with 0
the more polar the bond, the more like an ionic compound the molecule behaves.
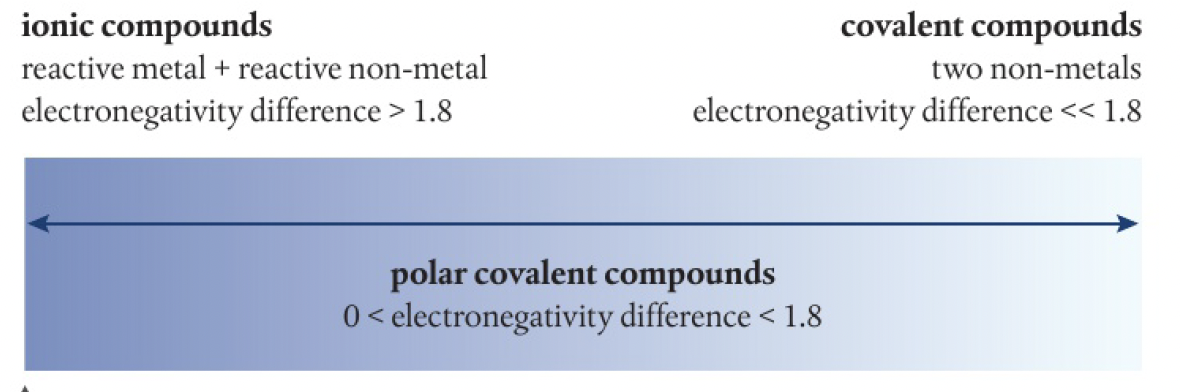
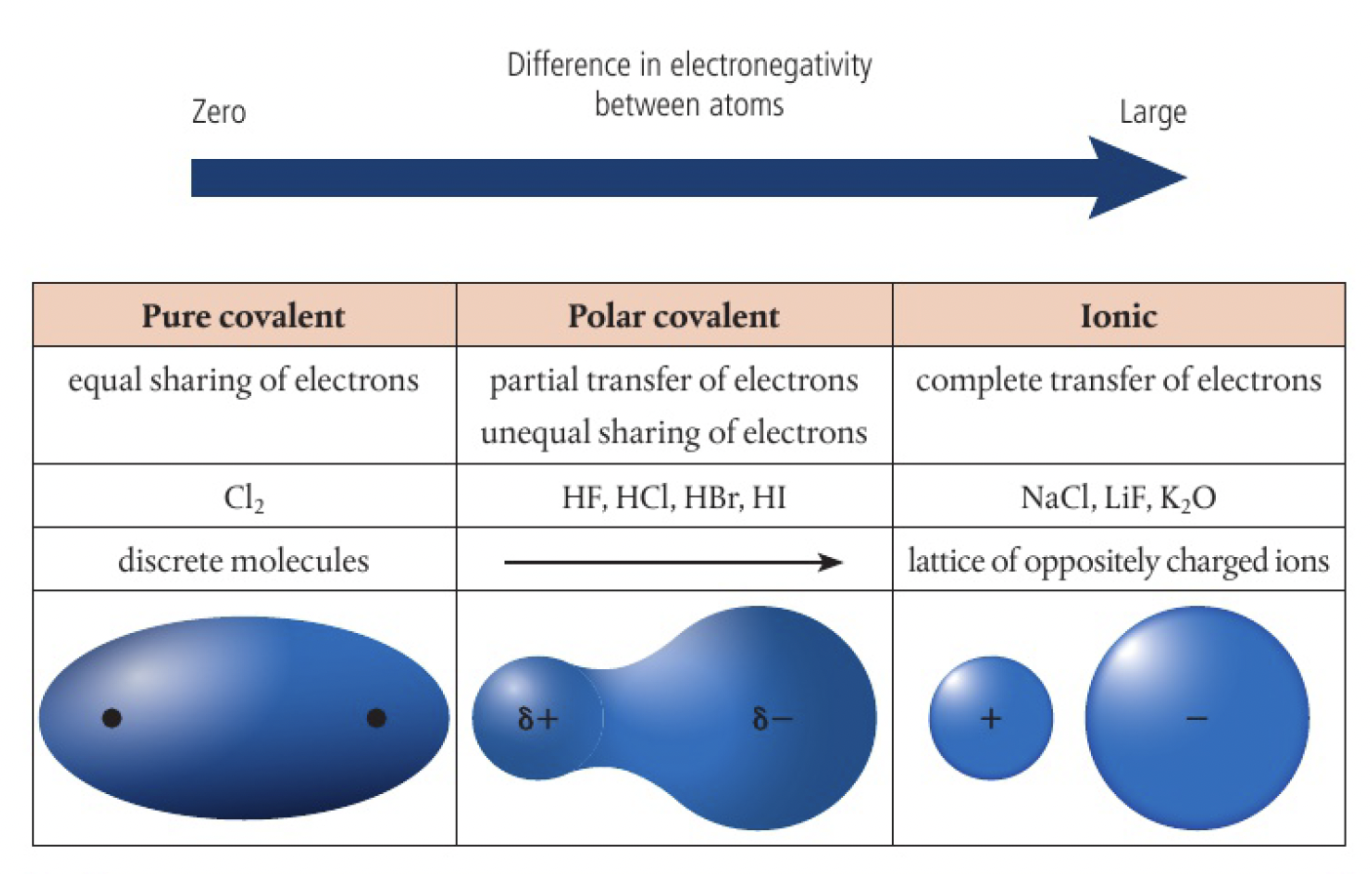
this explains some overlap in the properties of ionic and covalent substances. it is often more appropriate to describe substances using terms such as ‘predominantly’ covalent or ionic, or ‘strongly’ polar
challenge questions
- given the definition of ‘electronegativity’, why are the group 18 elements often not assigned a Pauling value?
the electronegativity of an atom measures its ability to attract electrons to itself in a covalent bond, however, group 18 elements typically do not react nor form chemical bonds, so a Pauling electronegativity value is not often assigned.
- oxygen is a very electronegative element with a value of 3.4 on the Pauling scale. can you determine the formula of a compound in which oxygen would have a partial positive charge?