Structure 3.1.10 - transition element complexes are coloured due to the absorption of light when an electron is promoted between the orbitals in the split
see 3.4.8 complex ions (HL)
see 3.1.6 oxidation states
transition metal ions in solution have a high charge density and attract water molecules which form coordination bonds to form complex ions
e.g.
6.png)
complexes are formed when a central ion is surrounded by molecules or ions with lone pairs - ligands, which form a coordination bond to the central metal ion.
the number of coordination bonds from the ligands to the central ion is called the coordination number
colour of transition metal ions
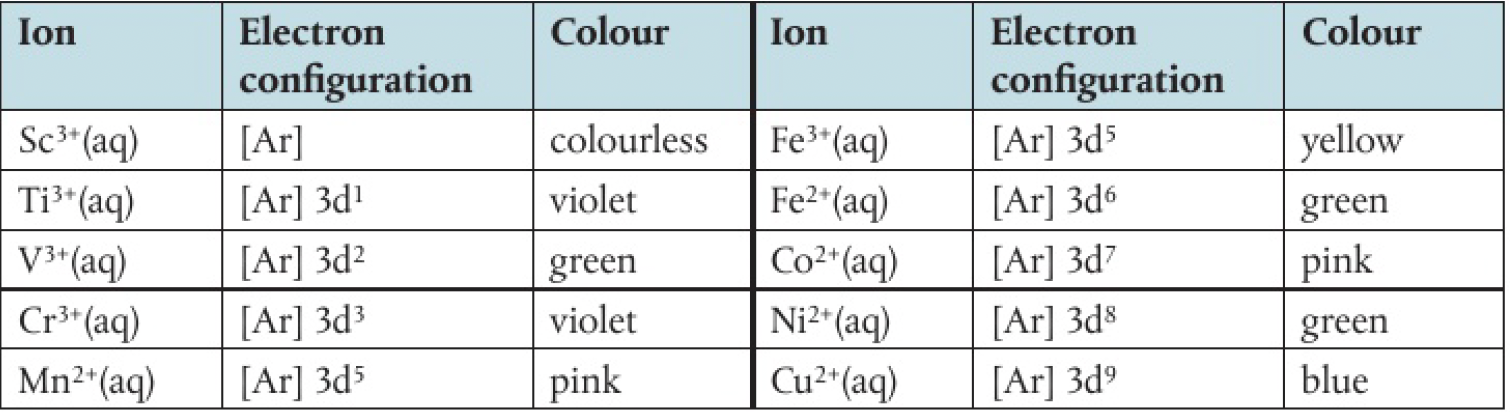
the colour of transition metal ions is related to the presence of partially filled
eg.
the
however, in a complex ion, the electric field produced by the ligand’s lone pair of electrons, the orbitals split into two levels.
the colour of light is dependent on the energy difference between the two levels, which can be changed by:
- the ligand present
- the oxidation number of number of d electrons present
- the nuclear charge of the ion
for example:
see 2.2.13 molecules with an expanded octet (HL) for molecular geometry
when light passes through a solution of
charge density of the ligand
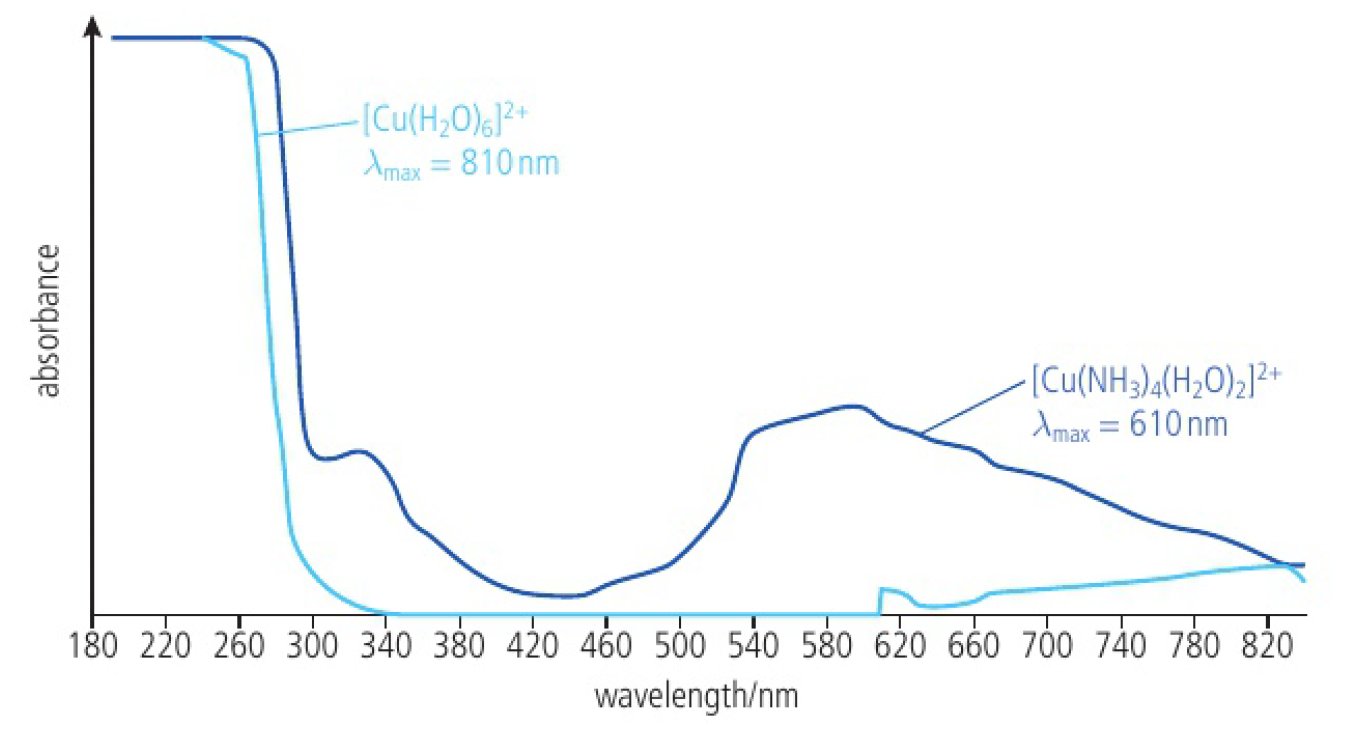
ˆUV-vis absorption spectrum of
ammonia has a greater charge density than water, so produce a larger split in the
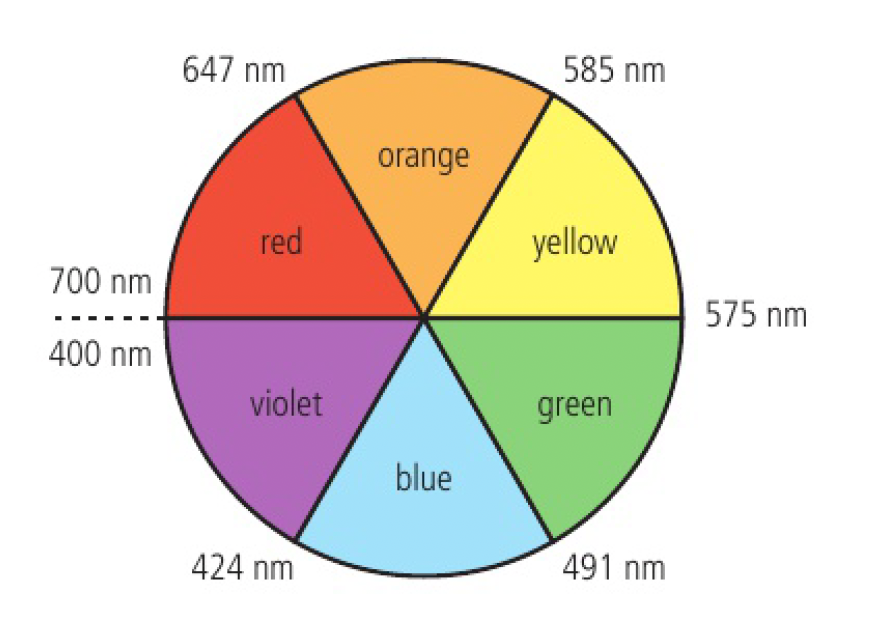
keep in mind that the visible range of light ranges from 400nm (red) to 700nm (violet), so only take values of max absorption in that range
the absorbance of light depends on the concentration
a calibration curve can be produced by measuring the absorbance at a wavelength for a series of standard solutions with known concentrations
- typically, 5 serial dilutions are done
challenge questions
interesting
transition metals’ complexes only have colour because of the partially filled d orbital split by ligands, which have different energies and can absorb different wavelengths of light. Zn, despite being in the d block, has a full d orbital, so it doesn’t really fit under the label of transition metal.
- cobalt chloride paper is used to indicate the presence of water. the colour changes from blue to pink as the ligands change from
ions to molecules. suggest why the two cobalt complex ions and are different colours.
the d sublevel splits due to the presence of the ligand’s lone pair of electrons. the energy difference between the two sets depend on the coordination number, which changes from six to four. thus, the amount of energy required to excite an electron changes and thus the wavelength of light absorbed and transmitted
- explain why the absorption spectra of gaseous atoms are made up of a series of lines whereas complex ions produce broad absorption bands
the spectrum of a complex ion is affected by surrounding ligands which can possess both vibrational and rotational energy, allowing the central ion to accept a wider range of frequencies. excess energy can be taken up by ligands as increased vibrational and rotational energy.
gaseous ions only absorb energy of the exact wavelength to move an electron to a higher energy atomic orbital