chem modelsofparticulatenatureofmatter
Structure 1.2.3 - Mass spectra are used to determine the relative atomic masses of elements from their isotopic composition.
note: operation details of the mass spectrometer will not be assessed.
see 3.2.8 mass spectrometry (HL)
- sample vaporised
- ionised by high-energy electrons which knock out an electron to produce a positively charged ion:
- the positively charged ions are attracted to a negatively charged plate and deflected by a magnetic field placed at right angles to the path
- the amount of deflection is inversely proportional to their mass/charge (m/z) ratio
single-charge ions with smaller mass are deflected more than heavier ions with the same charge
compounds may be fragmented by the high-energy electrons, resulting in fragmentation patterns
cue organic instrument analysis! Structure 3.2
relative values for the mass of atoms are used since the mass of elements are in the range of
mass spectra consist of x-axis of mass/charge (m/z) and y-axis for % abundance
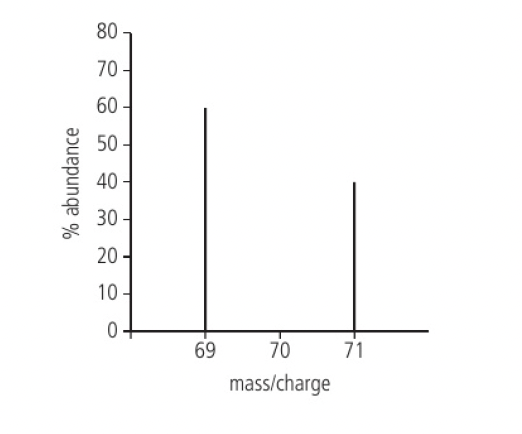
challenge questions:
- Suggest how the mass spectrum of gallium changes if the kinetic energy of the electrons used in ionising the sample in a mass spectrometer is significantly increased.
if the electrons were to have increased kinetic energy,