chem modelsofbondingandstructure
Structure 2.2.10 - chromatography is a technique used to separate the components of a mixture based on their relative attractions involving intermolecular forces to mobile and stationary phases.
components have different affinities for two phases, a stationary phase and a mobile phase, and are separated as the mobile phase moves through the stationary phase. the levels of solubility of each component in each of the phases is dependent on the intermolecular forces present.
paper chromatography
chromatographic paper and a solvent is used mainly for qualitative analysis
the paper, containing 10% water is the stationary phase. water is adsorbed by forming hydrogen bonds with the
as the mobile phase moves up, it dissolves the components of the mixture to different extents, carrying them at different rates. the components become spread out according to their different solubilities in the mobile and stationary phases.
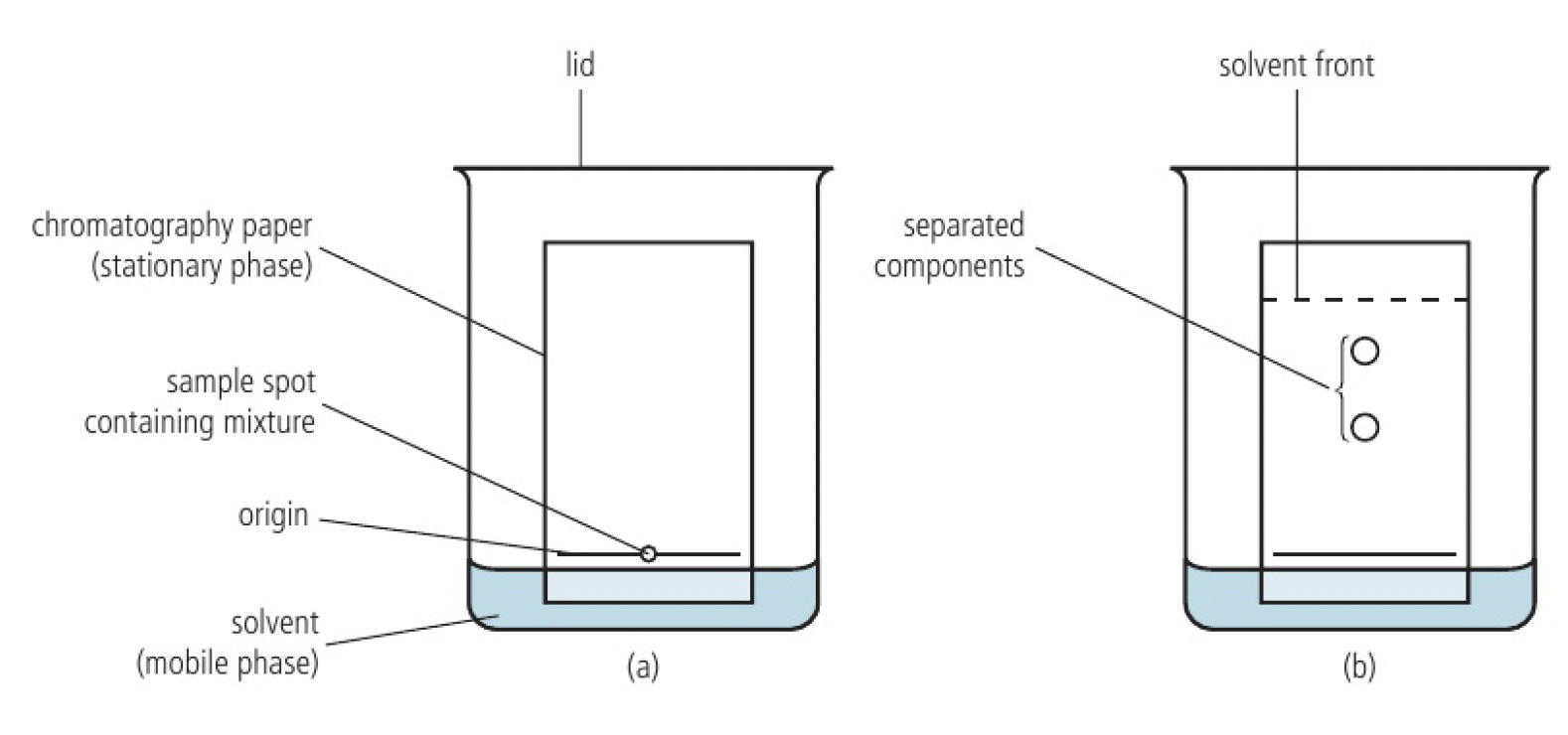
when the solvent almost reaches the top of the paper, its final position is marked and is known as the solvent front. the final result is known as a chromatogram.
the position of each component can be represented as an
thin layer chromatography
stationary phase is a uniform layer of silica (