chem whatarethemechanismsofchemicalchange
Reactivity 3.4.11 - alkenes readily undergo electrophilic addition reactions
see 2.2.15 sigma and pi bonds (HL)
see 3.4.5 electrophilic addition of alkenes
electrophilic addition reactions require the breaking of a
-
the carbon atoms are
hybridised, forming a planar triangular shape -
this is a fairly open structure that makes it relatively easy for incoming groups to attack
-
the
bond represents an area of electron density above and below the plane of the bond axis -
the
bond is attractive to electrophiles -
the electrons are less closely associated with the nuclei
reactions between reagents and alkenes are known as electrophilic addition reactions
addition reaction of halogens and alkenes
when ethene gas is bubbled through bromine at room temperature, the brown colour of the bromine fades as it reacts to form the saturated product of 1,2-dibromoethane.
reaction mechanism:
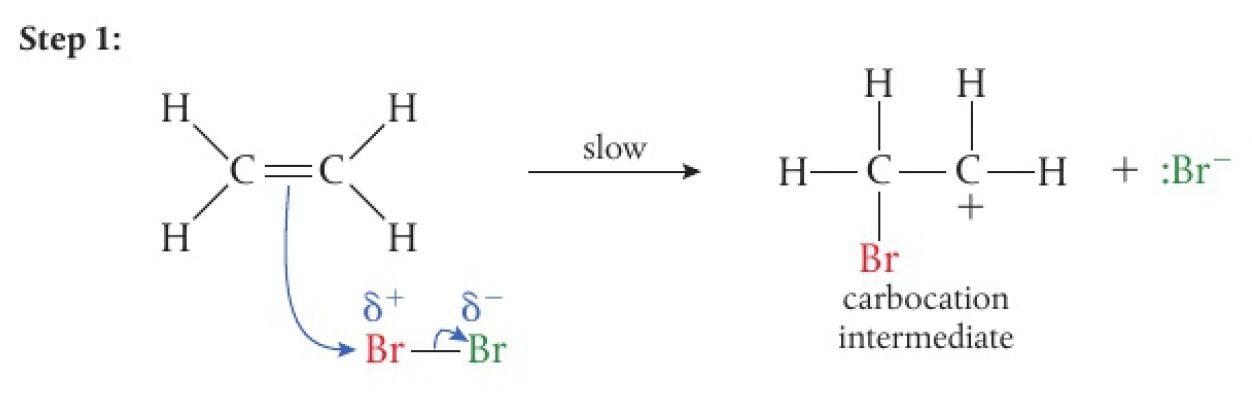
-
bromine becomes polarised as it approaches the electron-rich region of the alkene
- this is due to electron repulsion
- this creates a dipole
-
the electron-deficient bromine
then acts as the electrophile in the reaction -
the electrons in the
bond of the alkene are attracted to the electrophilic bromine -
this causes the
bond to break heterolytically
this step is slow, resulting in the formation of an unstable carbocation intermediate
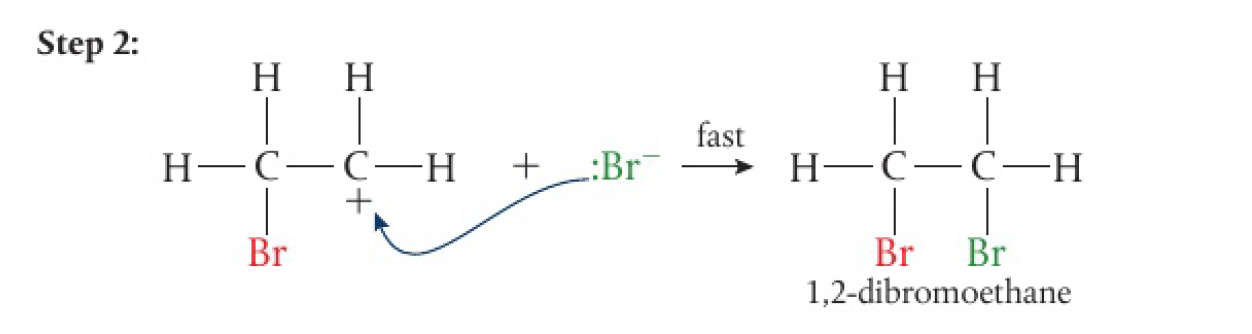
the unstable carbocation reacts rapidly with
the overall equation for the reaction is:
similar reactions take place with other alkenes such as propene:
evidence for this reaction mechanism comes from carrying out the same reaction with the additional presence of
- the products are
and - the carbocation formed in the first step is equally ready to combine with
or - the fact that no dichloro compound is ever formed confirms that the initial attack was by the electrophile
formed from
addition reaction of hydrogen halides and alkenes
- when ethene gas is bubbled through a concentrated aqueous solution of
- an addition reaction occurs fairly readily at room temperature, forming bromoethane
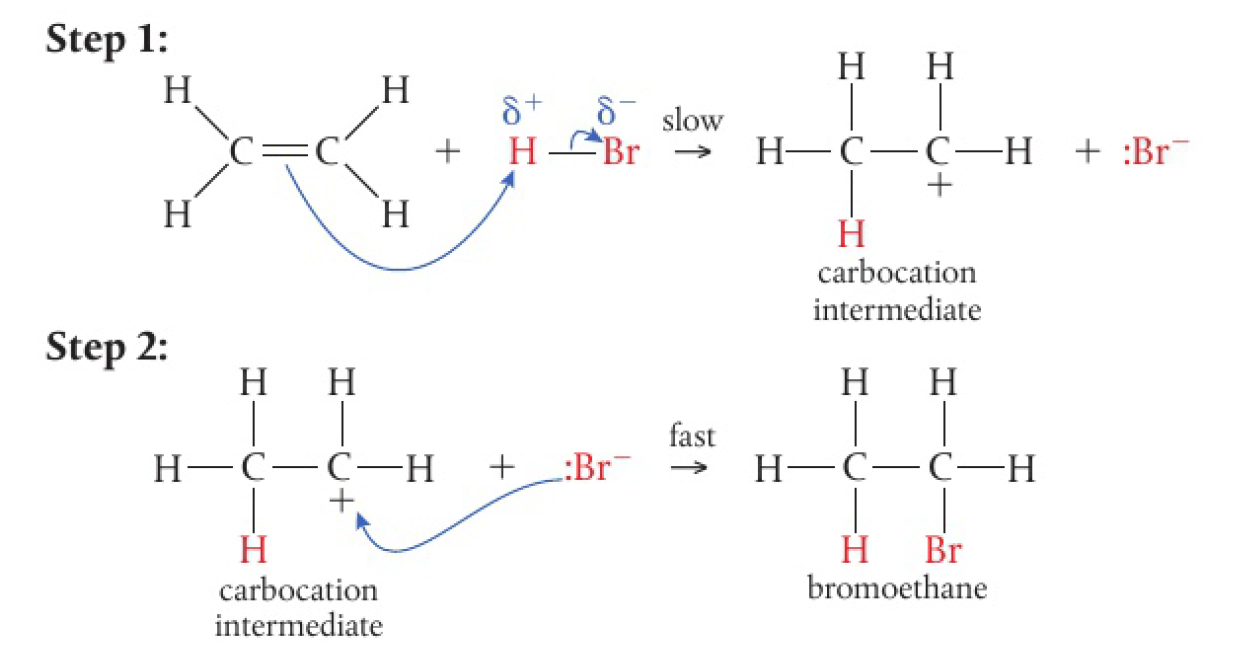
- the electrons in the
bond of the alkene are attracted to the electrophilic hydrogen - the
bond breaks heterolytically - the unstable carbocation intermediate that forms reacts quickly with
to form the addition product
the other hydrogen halides react similarly, but differ in terms of reactivity due to factors already discussed
addition reaction of water and ethene
- water is a weak electrophile and does not directly undergo addition reactions with alkenes
- the presence of strong acids can catalyse the reaction with
acting as the electrophile
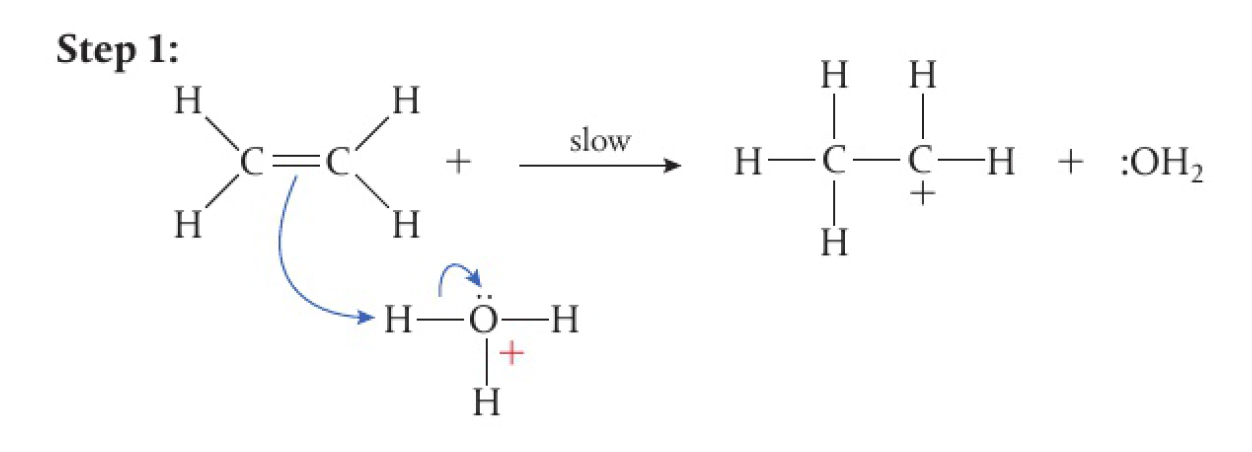
- the
electrons in the alkene are attracted to an electrophilic hydrogen - heterolytic fission of the
results in the formation of a carbocation intermediate and a water molecule
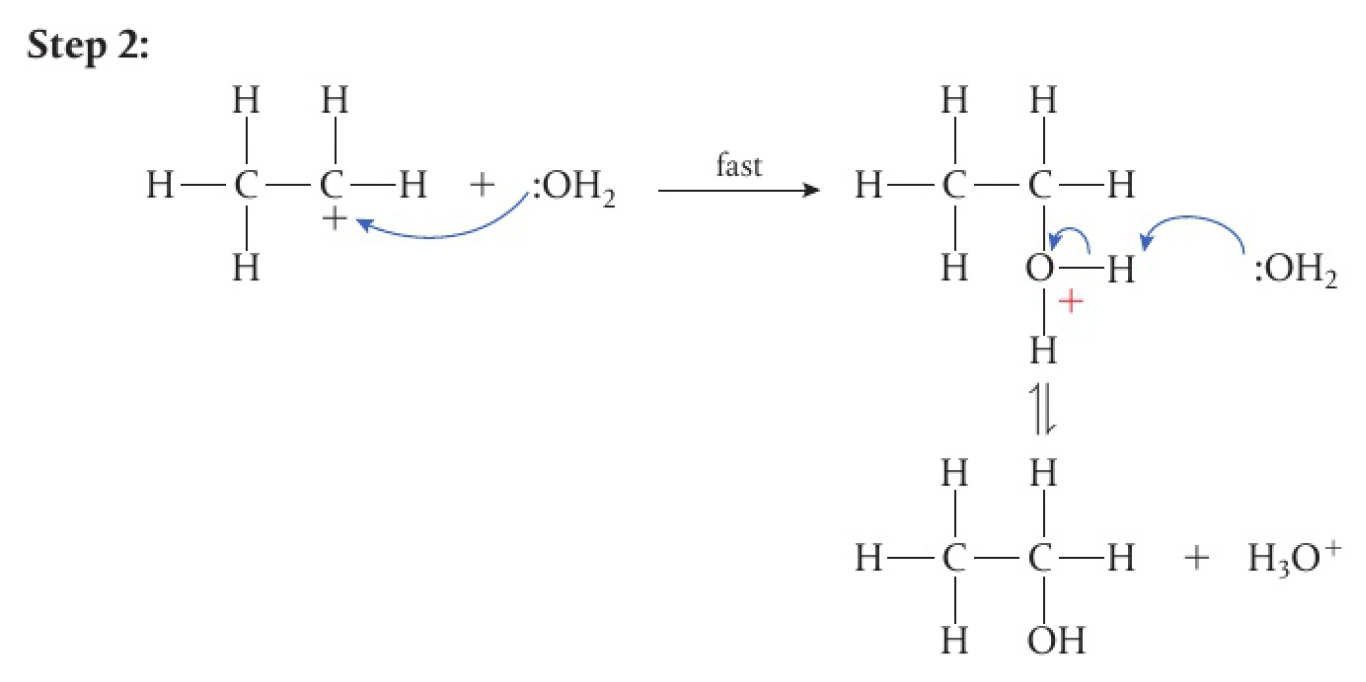
- water acts as a nucleophile, donating a lone pair of electrons to the positively charged carbon and forming a
bond - the product is positively charged, but an equilibrium reaction involving deprotonation occurs
- another water molecule acts as a base and the alcohol product is formed
- the
used in the first step is regenerated in the final step
challenge questions
- explain why hydrogen
is a very weak nucleophile and the addition reaction of alkenes with hydrogen (hydrogenation) requires metal catalysts such as , or and the reaction occurs at the surface of the catalyst. provide a general outline of the mechanism of this reaction and how it differs from the typical electrophilic substitution mechanism presented in this chapter.
- due to the very small size, the hydrogen atoms in
are not polarisable, meaning that does not readily form instantaneous dipoles, therefore a very weak electrophile. - when metal catalysts are used, the alkene and
are both adsorbed onto the surface of the metal. - this breaks the
bond and weakens the bond of the alkene. the atoms present on the metal surface can add to the alkene, forming the hydrogenated product
a typical electrophilic substitution mechanism does not require a catalyst and the
- use your knowledge of molecular geometries, intermolecular forces and melting points to explain why the hydrogenation of liquid vegetable oils can result in a solid product at room temperature.
vegetable oils contain long carbon chains with one or more
hydrogenation of vegetable oils result in the formation of saturated products due to the
the flexible alkyl chains are able to stack together more readily, resulting in the saturated products having stronger intermolecular forces and higher melting points