Reactivity 2.3.7 - the equilibrium constant and Gibbs energy change,
see 2.3.5 the reaction quotient, Q
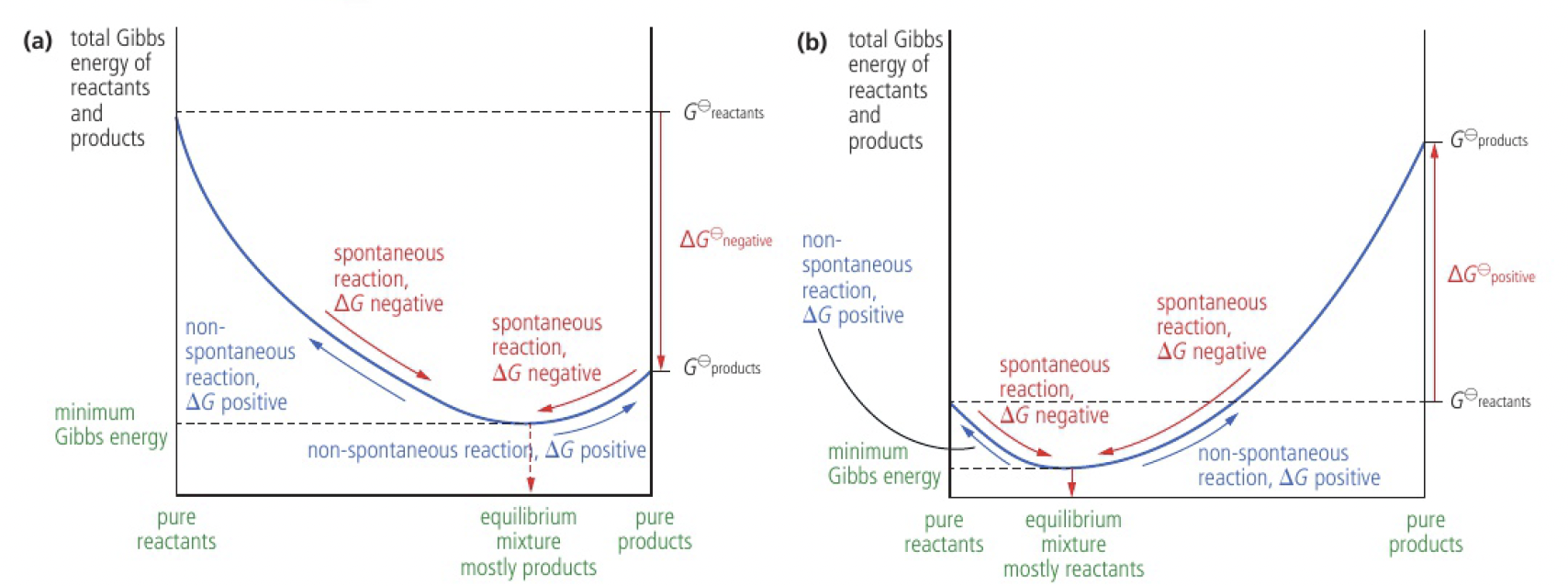
the sign of
calculation of
use
rate of reaction and equilibrium
the magnitude of
considering a reaction that is assumed to occur in a single step: (see 2.2.6, 2.2.7 and 2.2.8 reaction mechanisms)
only in this case, the rate laws can be written from the stoichiometry of the reaction:
where
the equilibrium constant expression for this reaction is:
at equilibrium, the rate of forward reaction is the same as the rate of backward reaction, so:
the equilibrium constant is the ratio of the rate constants of forward and backward reactions.
- if
is large and reaction progresses towards completion - if
is small and the reaction barely takes place - increasing the concentration of reactants increases the rate of the forward reaction. following le chatelier’s principle,
stays constant - adding a catalyst increases the values of
and by the same factor, so stays constant - from the Arrhenius equation,
, for the forward and backward reactions is different, so they are differently affected by temperature - for an endothermic reaction where
, increase in temperature has a greater effect on increasing than , so increases as temperature increases
- for an endothermic reaction where