chem whatdriveschemicalreactions
Reactivity 1.1.4 - the standard enthalpy change for a chemical reaction,
standard enthalpy change,
- pressure of 100
- 1
for all solutions - all substances in standard states
temperature is not part of the definition of standard state, but 298
thermochemical equations
combustion of methane
shorthand of expressing that one mole of methane gas reacts with two moles of oxygen gas to give one mole of gaseous carbon dioxide and two moles of liquid water and releases 890
specific heat capacity
- Q is the heat added
- m is the mass of the object
- c is the specific heat capacity
is the temperature change
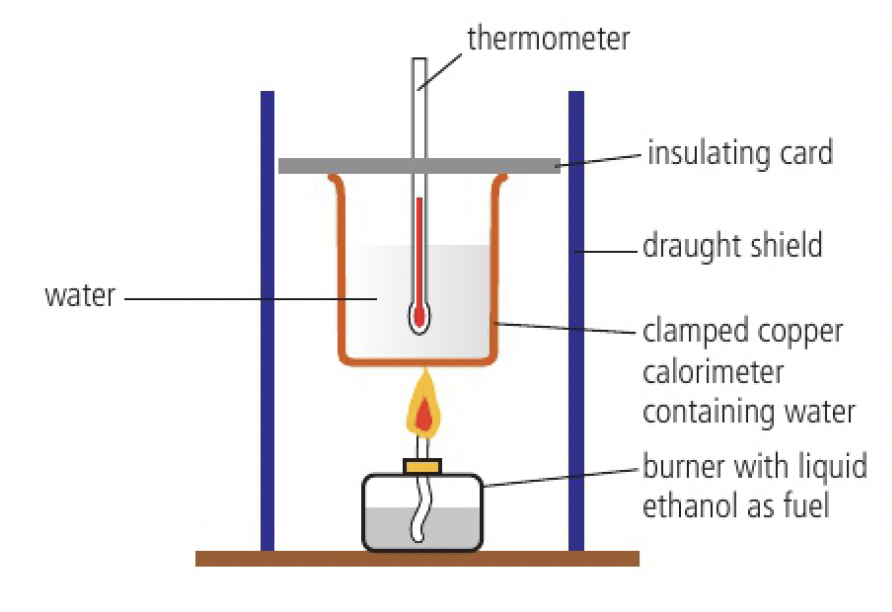
measuring enthalpy change
a calorimeter can be used to measure the enthalpy change of combustion,
enthalpy changes of a reaction in solution
the enthalpy changes of a reaction in solution can be calculated by carrying out the reaction in an insulated system. the heat released or absorbed can be measured from the temperature change in the water which is acting as a solvent.
this is typically conducted in a polystyrene cup, which is an insulator
one of the largest sources of error in experiments conducted on polystyrene cups is heat loss to the surroundings:
- heat is lost as soon as the temperature rises above the temperature of the surroundings, so the maximum recorded temperature would be lower than the true value in a perfectly insulated system
- allowance for heat loss can be made by extrapolating the cooling section of the graph of temperature against time of the cup. the following assumptions can be made:
- no heat loss from the system
- all the heat goes from the reaction to the water
- the solution is dilute
- water has a density of 1.00
for an exothermic reaction,
challenge questions
- explain the particularly high specific heat capacity of water.
water has a high heat capacity as when heat energy is added to water, some of the energy is needed to break the hydrogen bonds, so less energy is available to increase the kinetic energy. the presence of hydrogen bonds reduces the temperature increase and increases the specific heat capacity
-
a piece of brass is held in the flame of a Bunsen burner for several minutes. the brass is then quickly transferred into an aluminium calorimeter which contains 200.00g of water
determine the temperature of the Bunsen flame from the follow data
| m(water) | 200.00 |
| m(brass) | 212.10 |
| m(aluminium calorimeter) | 80.00 |
| c(brass) | 0.400 |
| c(Al) | 0.900 |
| initial temp of water | 24.5 |
| final temp of water | 77.5 |
