chem whatarethemechanismsofchemicalchange
Reactivity 3.4.13 - electrophilic substitution reactions include the reactions of benzene with electrophiles
- benzene is resonance stabilised
- 3
bonds with 3 delocalised electrons - this minimises the repulsion between the
electrons, so the benzene molecule is stabilised compared to regular alkenes
despite the high unsaturation, benzene does not behave like alkenes in its characteristic reactions:
- the unusual and highly stable aromatic ring determines that substitution is favoured rather than addition
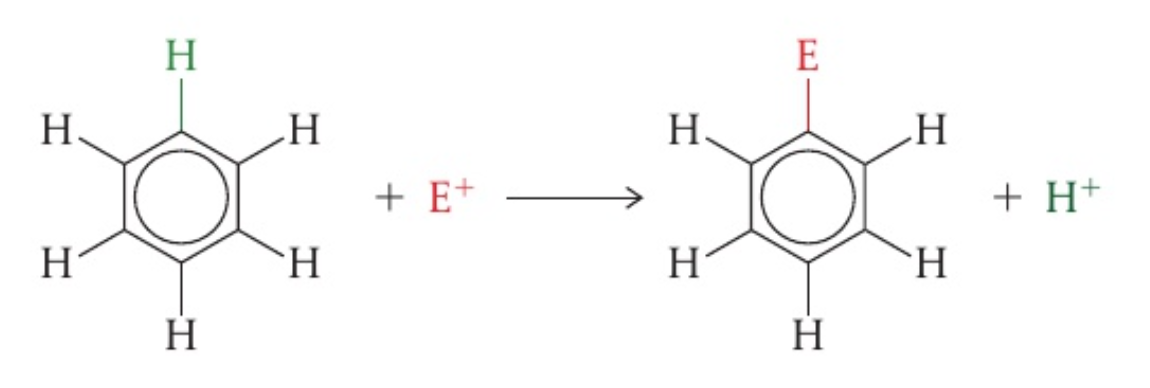
electrophilic substitution reactions of benzene
- benzene is attractive to electrophiles because its ring is a region of high electron density
- the delocalised cloud of
electrons attracts electrophiles and can form a new bond as a hydrogen atom is lost
-
the mechanism follow a similar path with different electrophiles
-
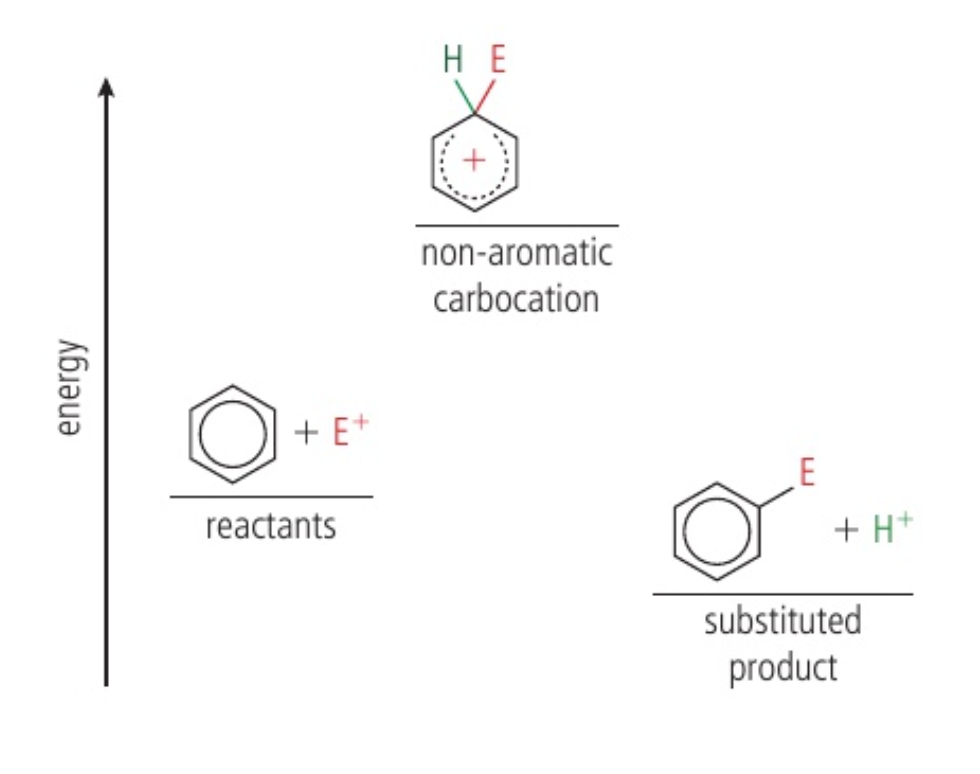
the reaction has a high activation energy, so it proceeds rather slowly
-
the first step in the mechanism where an electron pair from benzene is attracted to the electrophile leads to a disruption of the delocalised
system -
the unstable carbocation intermediate that forms has both the entering atom or group and the leaving hydrogen temporarily bonded to the ring
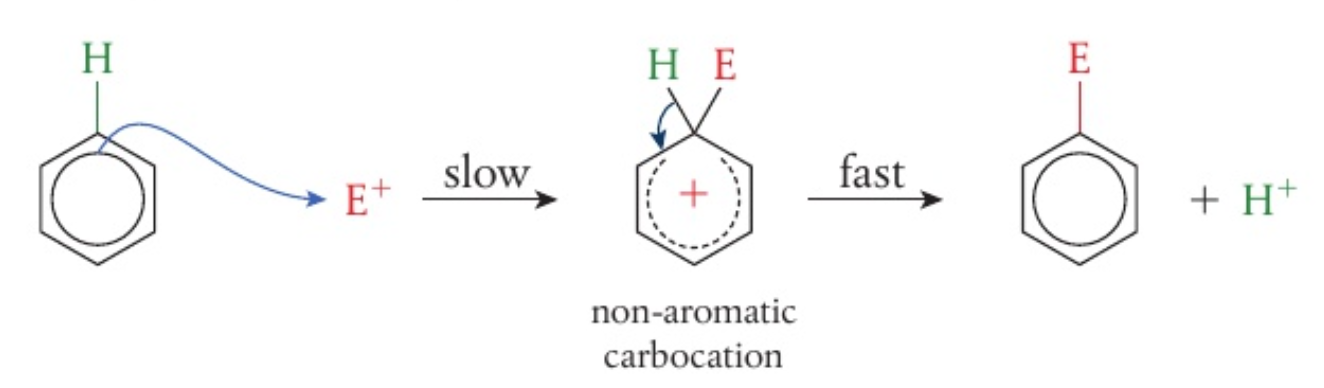
- the incomplete circle inside the ring shows that the delocalised
system has been disrupted - the positive charge is distributed over the carbon atoms in the ring (shown by the
bond results in 2 electrons from the bond moving to regenerate the aromatic ring and the formation of a neutral substitution product - this product is more stable
these reactions can be used with different electrophiles to introduce different functional groups
nitration of benzene
c1ccccc1(N(=O)[O])-
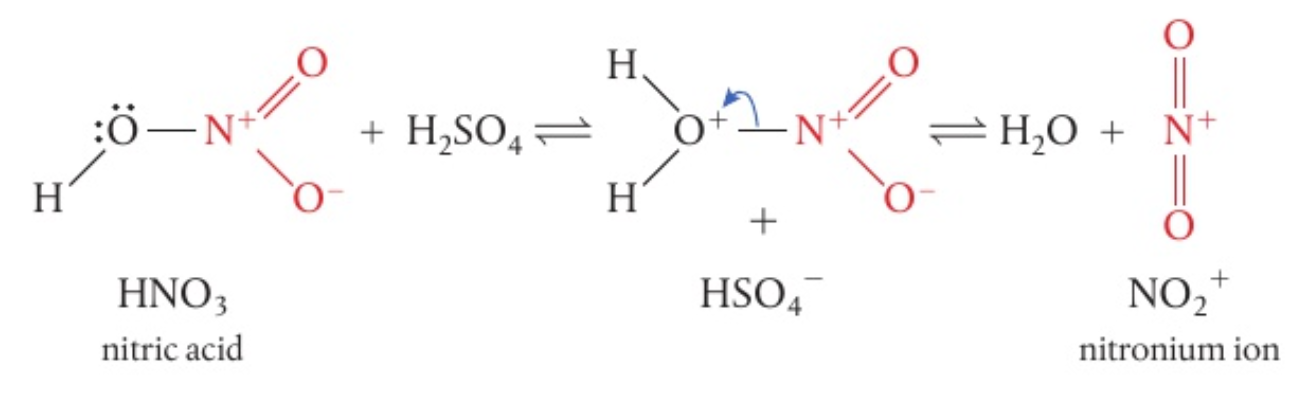
the electrophile for the reaction is
, the nitronium ion -
is generated a nitrating mixture - concentrated
and concentrated at 50 C
- concentrated
-
as the stronger of the two acids,
protonates -
the initial product formed then loses a molecule to produce the electrophile
is a strong electrophile and attracts the electrons of the benzene ring to form the carbocation intermediate - loss of a proton from this intermediate leads to reformation of the arene ring in the product, appearing as a yellow oil
- the hydrogen ion released reacts with the base
to reform
the overall reaction is:
c1ccccc1.[O-]N(=O)(O[H])>S(O[H])(O[H])(O)(O)>c1ccccc1N(=O)[O].[H]O[H]most of the nitrobenzene produced by this reaction is converted to phenylamine
c1ccccc1Nchallenge questions
- relative to benzene, phenylamine,
the electron density in the ring:
phenylamine > benzene > nitrobenzene
the electronegativity of the
the
the
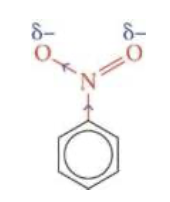
the electrons in its double bond conjugate with the