chem modelsofbondingandstructure
Structure 2.4.4 - polymers are large molecules, or macromolecules, made from repeating subunits called monomers
see 2.4.5 addition polymers
see 2.4.6 condensation polymers (HL)
see 3.2.2 functional groups and classes of compounds
some small molecules, known as monomers, are able to react to form a linked chain held together by covalent bonds, known as a polymer or macromolecule.
polymers are shown as a repeating unit, with open bonds at each end
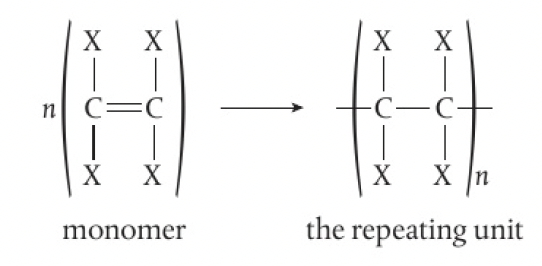
there are natural polymers such as starch, protein, and DNA and synthetic polymers such as plastics
plastics have widespread uses due to their:
- light weight
- low reactivity
- water resistance
- (strength)
non-biodegradable: not able to be broken down by natural processes
biodegradable: can undergo breakdown by microorganisms into end products found in nature, not harmful to environment
biodegradable plastics might be plant based, containing starch, lignin or cellulose, and often produced from corn. its breakdown products are
compostable plastics break down along with food and garden waste in the specific conditions found in a compost pile