chem whatdriveschemicalreactions
Reactivity 1.4.4 - as a reaction approaches equilibrium,
so far, only reactions in which all reactants are converted into products have been considered
consider a reaction:
- where an ideal gas reactant is converted into an ideal gas product
- where no bonds broken or formed during the reaction, so
- where is no change in entropy, so
- thus,
consider the same reaction in the reverse direction.
the outcome is identical, neither reaction is favoured, so an equilibrium mixture with equal amounts of reactant and product is likely to be produced
- a mixture of reactant and product has higher entropy than pure samples of reactant or product
- the total entropy reaches a maximum when there are equal amounts of reactant and product
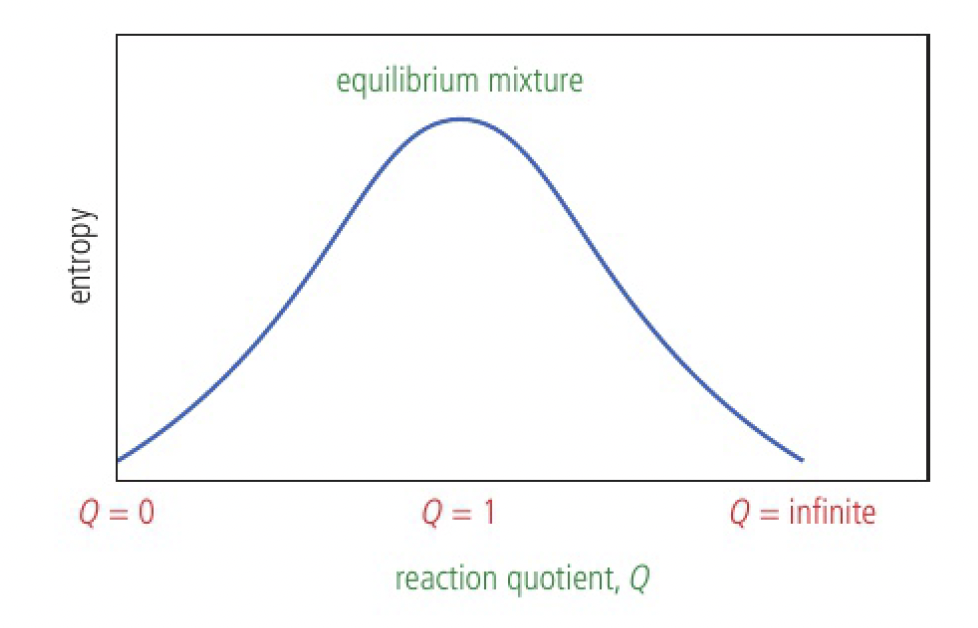
the entropy is due to mixing. the entropy of both pure states will be the same.
consider the case of 6 reactants
the entropy increases to a maximum at
the extent of the reaction is characterised by the ratio of products to reactants, the reaction quotient, Q.
in this hypothetical example, the system reaches a minimum of Gibbs energy at equilibrium:
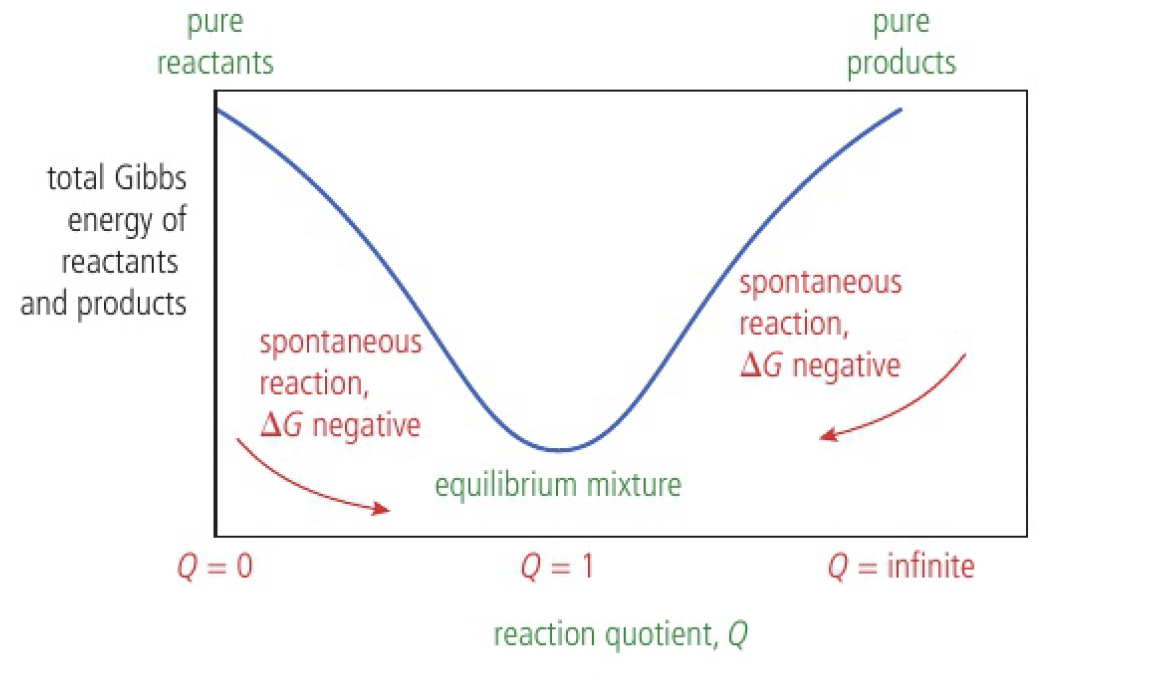
equilibrium mixture when
- at the start, the total Gibbs energy of the reactants is greater than that of the products, so the reaction proceeds in the forward direction
- as the reaction continues, the Gibbs energy of the system decreases until it reaches equilibrium.
- once equilibrium is reached, all possible changes would lead to an increase in Gibbs energy and a decrease in the total entropy of the universe. so no change occurs
the position of equilibrium corresponds to a mixture with more products than reactants
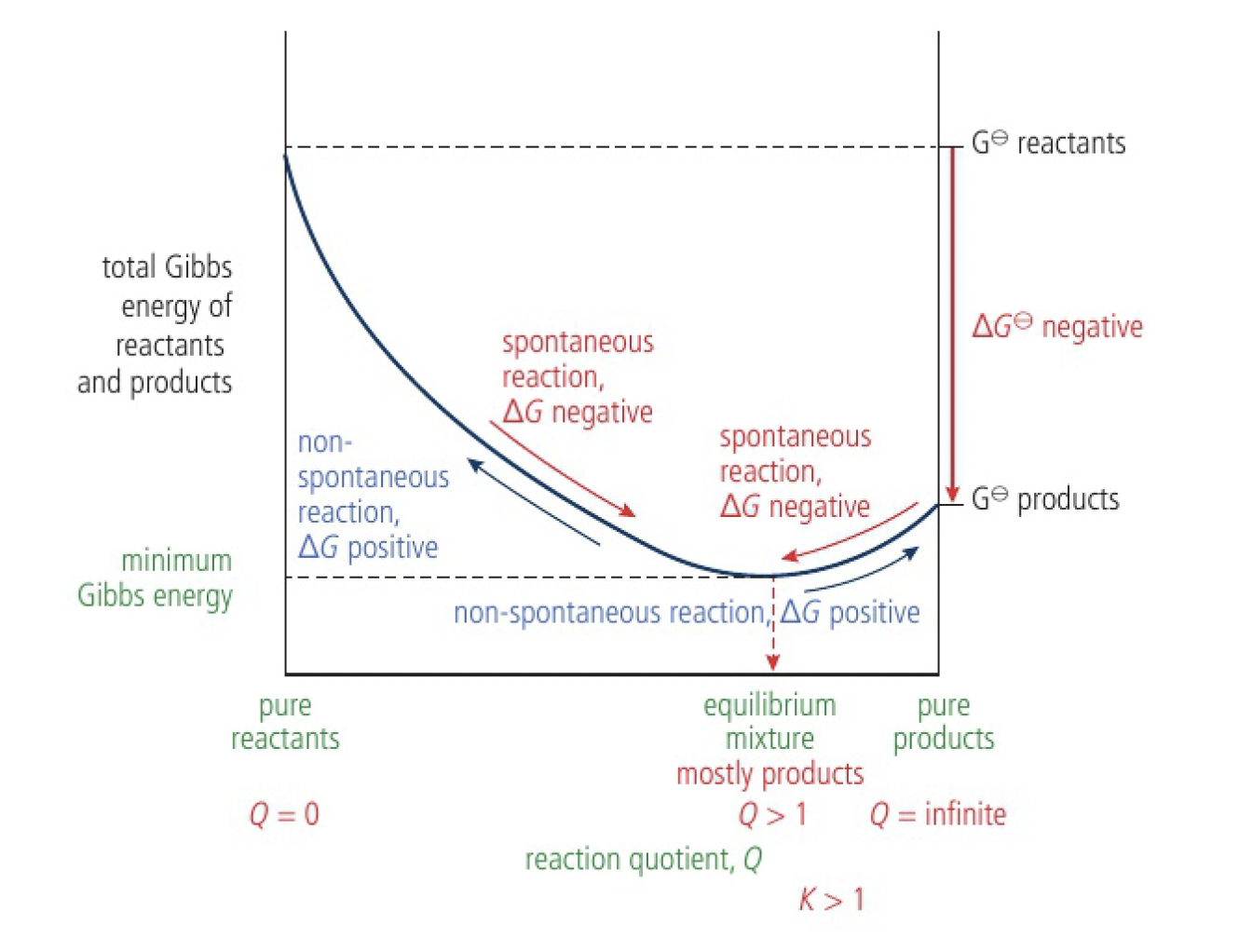
since
Important
the Gibbs energy of the pure reactants is greater than the Gibbs energy of the pure products. the equilibrium mixture consists largely of products,
equilibrium mixture when
similar arguments can be used to explain why the equilibrium mixture contains more reactants than products when
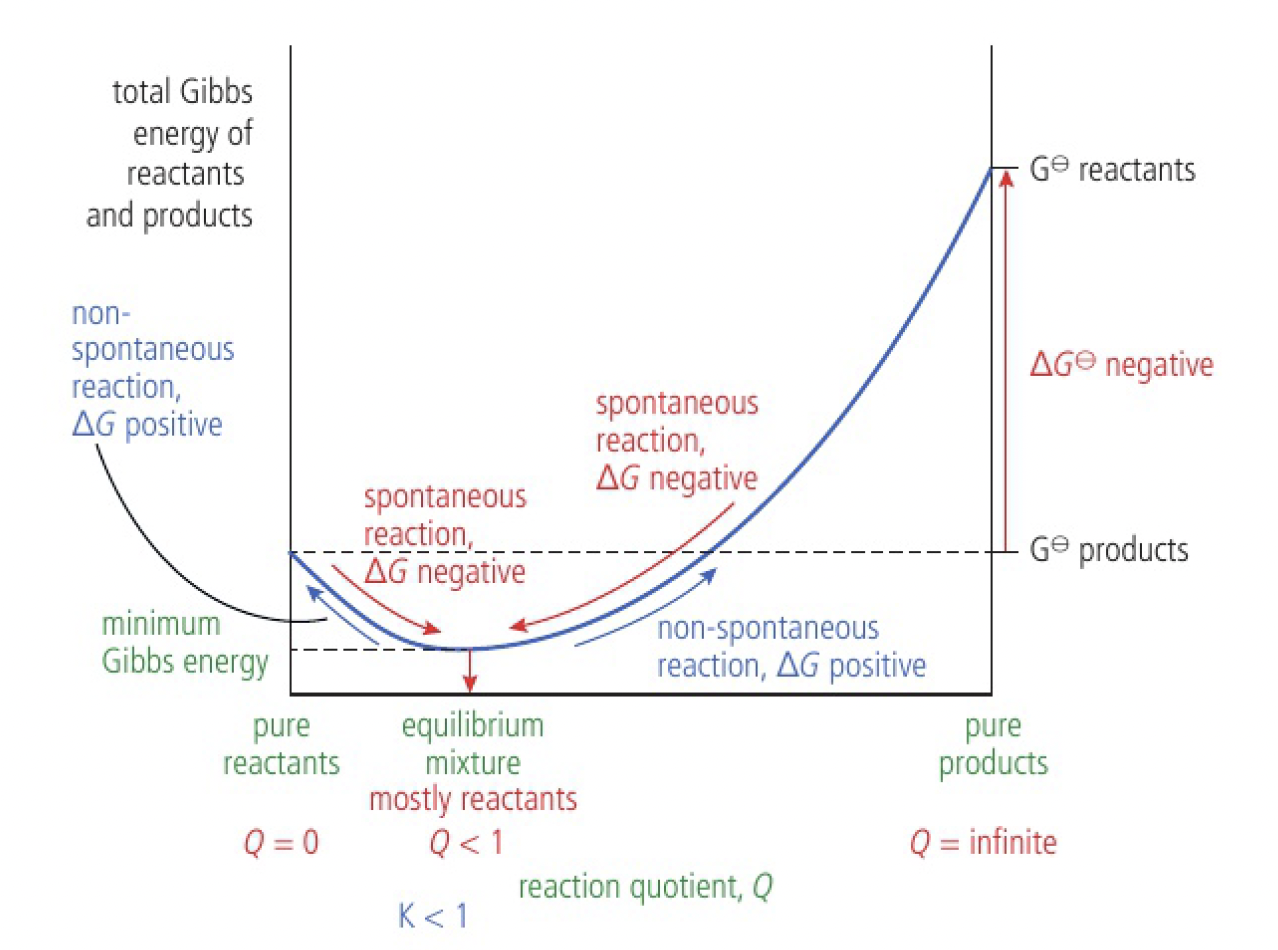
explained using similar arguments as above
Important
the Gibbs energy of the pure reactants is less than the Gibbs energy of the pure products. the equilibrium mixture consists largely of reactants,
the equilibrium constant
the position of equilibrium is related to two terms:
, the equilibrium constant , the change in Gibbs energy
| equilibrium mixture | K | |
|---|---|---|
| negative | mainly products | |
| positive | mainly reactants | |
| 0 | significant amounts of both reactants and products |
more formally:
- where
is the standard Gibbs energy change of the reaction is the gas constant is the absolute temperature is the equilibrium constant
note thathas the same units as
this can be used in situations where the equilibrium constant is difficult to measure directly, or where some of the components are too small to measure
| extent of reaction | |
|---|---|
| no reaction | |
| partial reaction producing equilibrium mixture | |
| partial reaction producing equilibrium mixture | |
| partial reaction producing equilibrium mixture | |
| complete reaction |
challenge questions
- consider the enthalpy and entropy changes for the same reaction in the reverse direction:
the original reaction:
-
an ideal gas reactant is converted into an ideal gas product
-
there are no bonds broken or formed during the reaction, so
-
there is no change in entropy, so
-
thus,
what does this suggest about the direction of change?
the outcome is identical, neither reaction is favoured, so an equilibrium mixture with equal amounts of reactant and product is likely to be produced
- explain the shape of the graph in figure 4.

the entropy is due to mixing. the entropy of both pure states will be the same.
consider the case of 6 reactants
the entropy increases to a maximum at
-
for the reaction
find a mathematical function of
which gives values of consistent with the previous discussion (question 2)
for:
where