chem modelsofparticulatenatureofmatter
Structure 1.1.3 - temperature in kelvin is a measure of average kinetic energy (
absolute temperature is directly proportional to the average kinetic energy of its particles
0K = -273.15
- this is the lowest temperature attainable, as this is when all movement stops
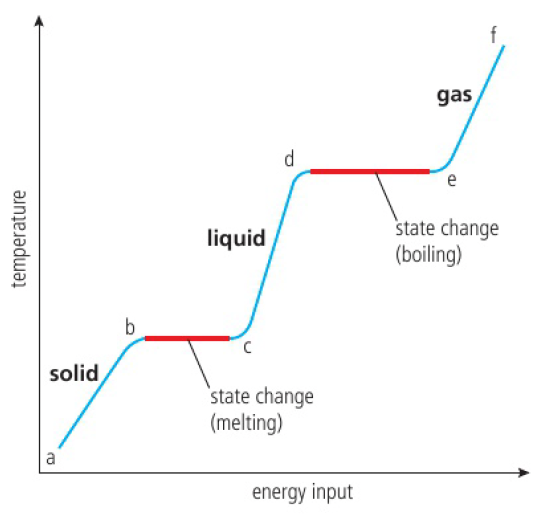
a-b
as the solid is heated, vibrational energy increases, temperature increases.
b-c
the vibrations are energetic enough for particles to move away from fixed positions and form a liquid. the temperature remains constant as the energy is used to break inter-particle forces.
c-d
as the liquid is heated, vibrational energy increases, temperature increases.
d-e
this is the boiling point. there is enough energy to break all inter-particle forces and form a gas.
note that this state change requires more energy than solid-liquid as all inter-particle forces must be broken.
temperature remains constant as energy is used to break inter-particle forces
e-f
as the gas is heated under pressure, the kinetic energy of its particles rise, so the temperature continues to rise too
melting (b-c) and boiling (d-e) are endothermic processes as energy is taken from the environment
freezing (c-b) and condensation (e-d) are exothermic processes as energy is given to the environment as inter-particle forces bring the particles closer together
check out 1.1.1 chemical reactions involve heat transfers for more detail
challenge questions
- Which physical properties determine the gradient of the lines for the different states in figure 5?
the mass and specific heat capacity determines the gradient of the lines.