chem whatarethemechanismsofchemicalchange
Reactivity 3.4.5 - alkenes are susceptible to electrophilic attack because of the high electron density of the carbon-carbon double bond. these reactions lead to electrophilic addition
- alkenes have the general formula
and are unsaturated hydrocarbons with a bond
- alkenes are more reactive than alkanes as the double bond has a higher electron density
- the reaction occurs at the double bond
- the pi bond is selectively broken, creating 2 new bonding positions
addition of water
- hydration converts the alkene into an alcohol
- water is a poor electrophile
- hydration requires the use of a strong acid catalyst
- typically
addition of halogens
- halogens react with alkenes to produce dihalogeno compounds
- these reactions happen quickly at room temperature
- accompanied by loss of colour of reacting halogen
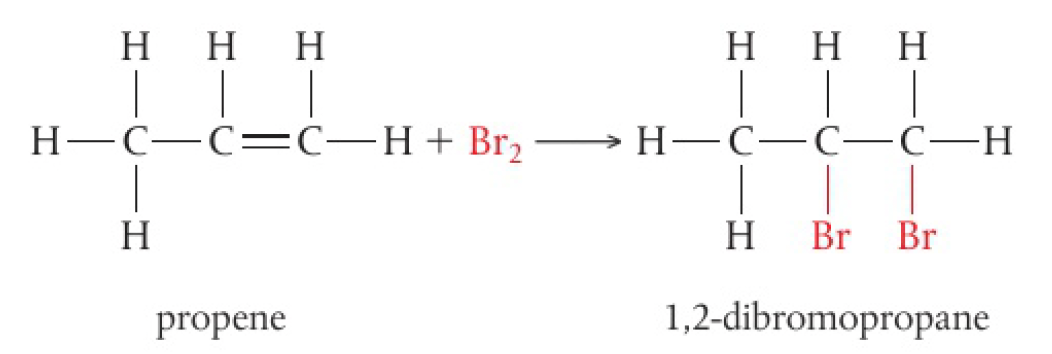
- bromine water is often used to distinguish between alkanes and alkenes
- alkanes react with bromine water through a radical mechanism (requiring UV light to initiate the reaction)
- alkenes react readily with
addition of halogen halides
- these reactions take place rapidly at room temperature
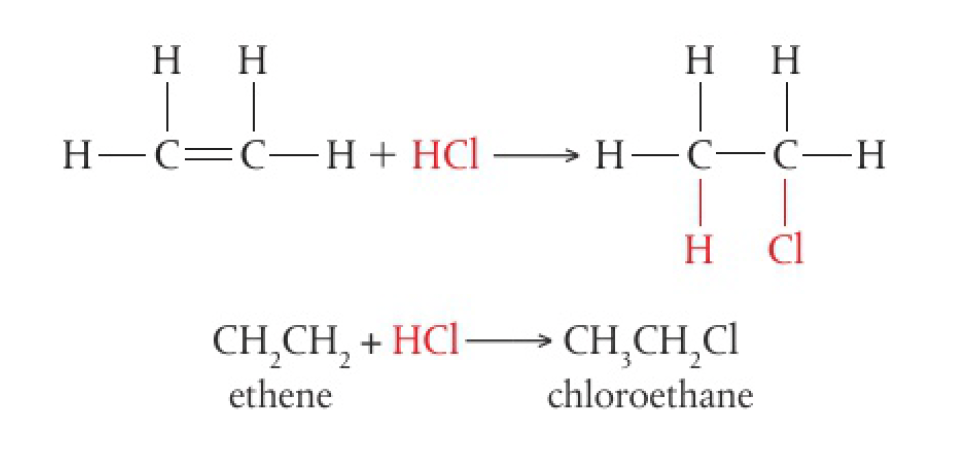
all hydrogen halides are able to react in this way, but the reactivity is in the order decreasing strength of the
alkenes can react with specific electrophiles to make different classes of organic compounds, such as:
- alcohols
- halogenoalkanes
- dihalogenoalkanes
these products can be further converted into other classes of compounds, making alkene very useful starting molecules in organic synthetic pathways