chem modelsofbondingandstructure
Structure 2.2.13 - some atoms can form molecules in which they have an expanded octet of electrons
see 2.2.4 the valence shell electron pair repulsion (VSEPR) model
when the central atom is an element from period 3 or further down, there exist compounds with more than eight electrons around the central atom - an expanded octet
- due to d orbitals in valence shell having close energy values to p orbitals
promotion of electrons from 3p to 3d can allow additional electron pairs
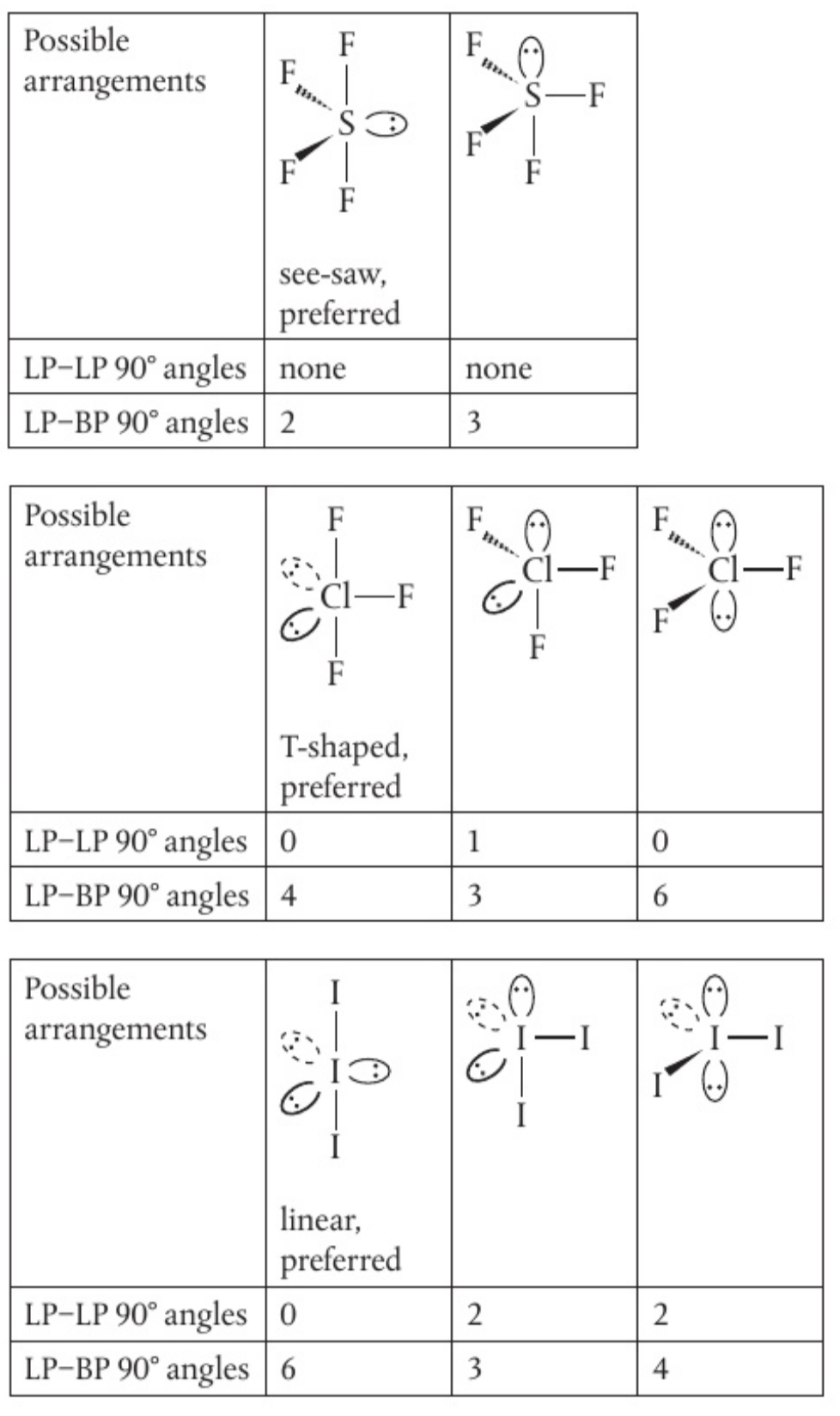
five electron domains
determining the shape:
- find arrangement with lowest number of lone pair to lone pair 90
angles - find arrangement with lowest number of 90
angles between lone pairs and bonding pairs - prefer lowest number of LP-LP 90
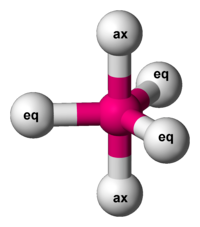
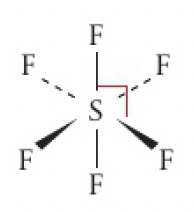
0 non-bonding pairs: triangular bipyramidal
1 non-bonding pair: unsymmetrical tetrahedron or see-saw.
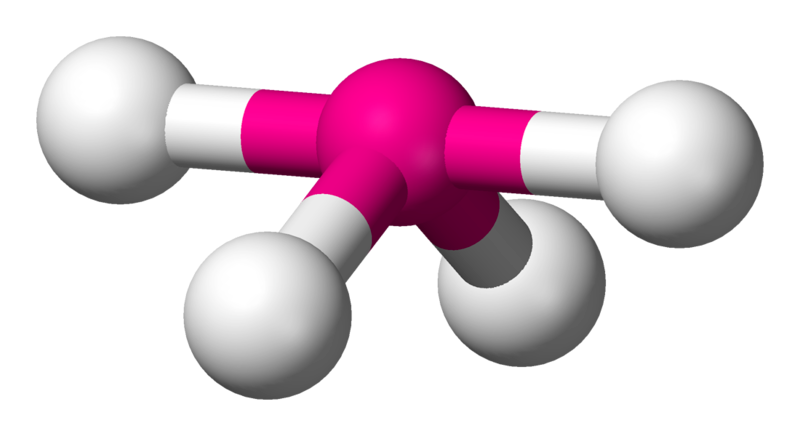
distortion from greater repulsion of non-bonding electrons
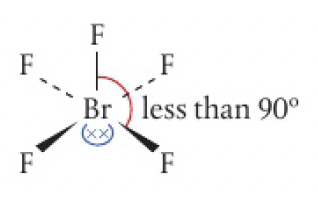
2 non-bonding pairs: T-shaped
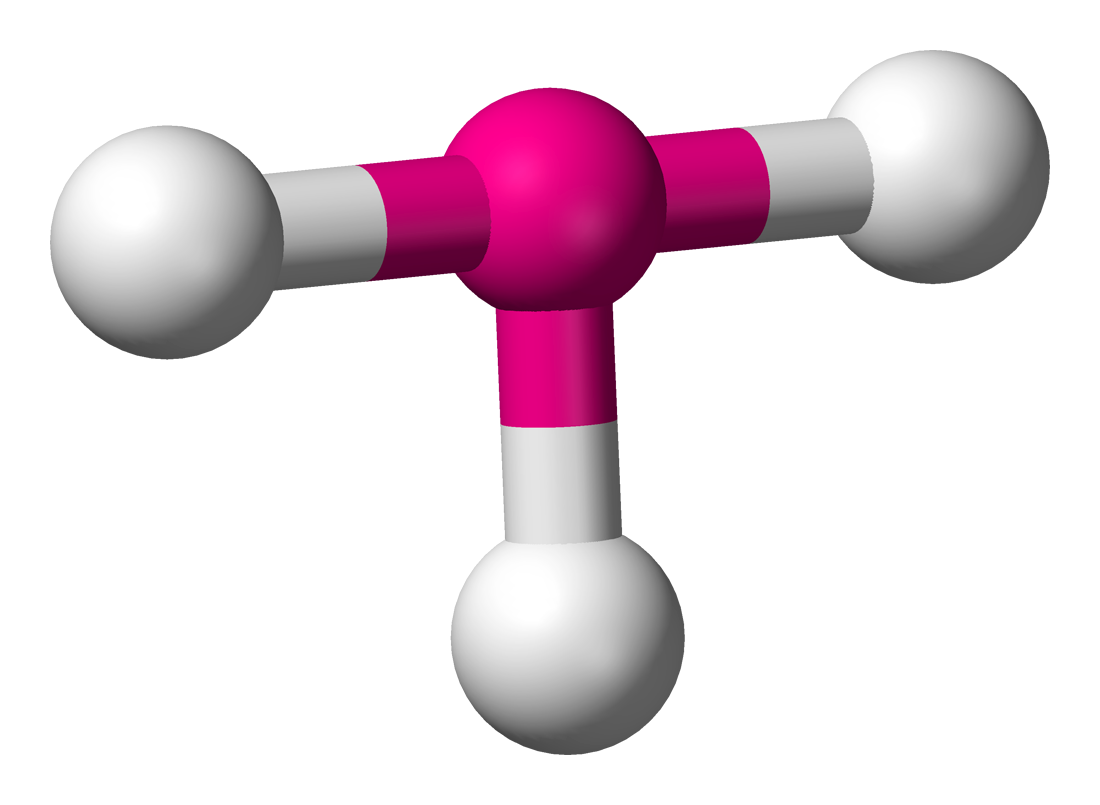
- bond angles less than 90
- electrons located in equatorial position to minimise repulsion
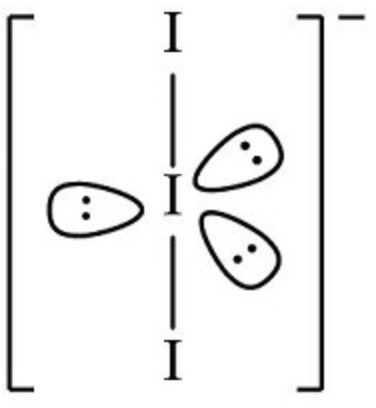
3 non-bonding pairs: linear
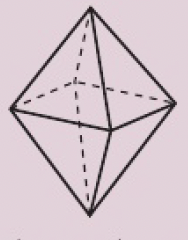
six electron domains
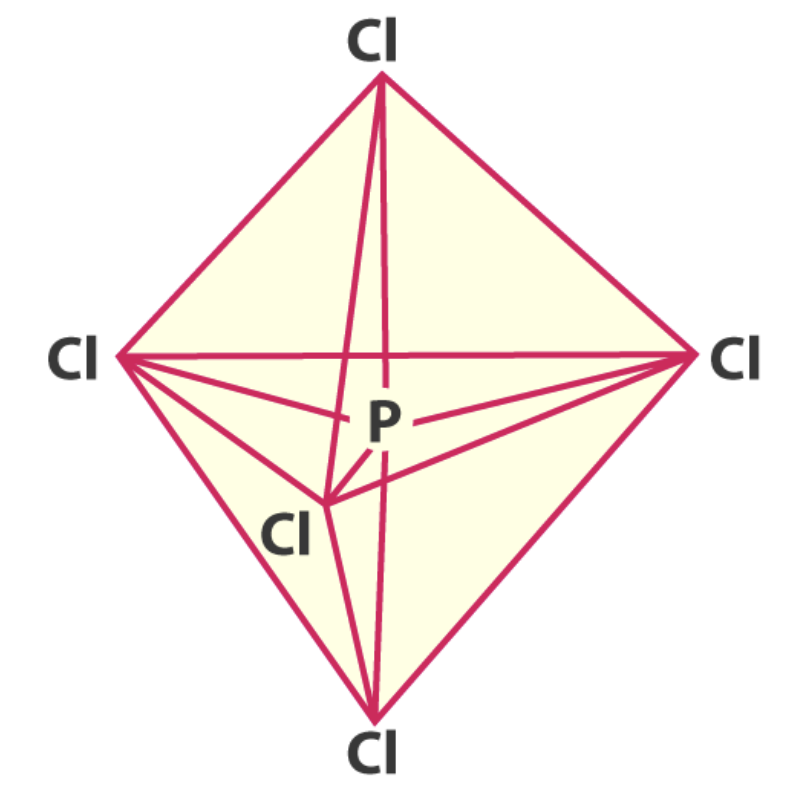
0 non-bonding pairs: octahedral
- eg
one non-bonding pair: square pyramidal shape
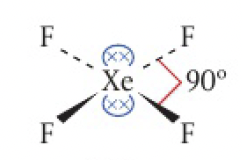
two non-bonding pairs: square planar
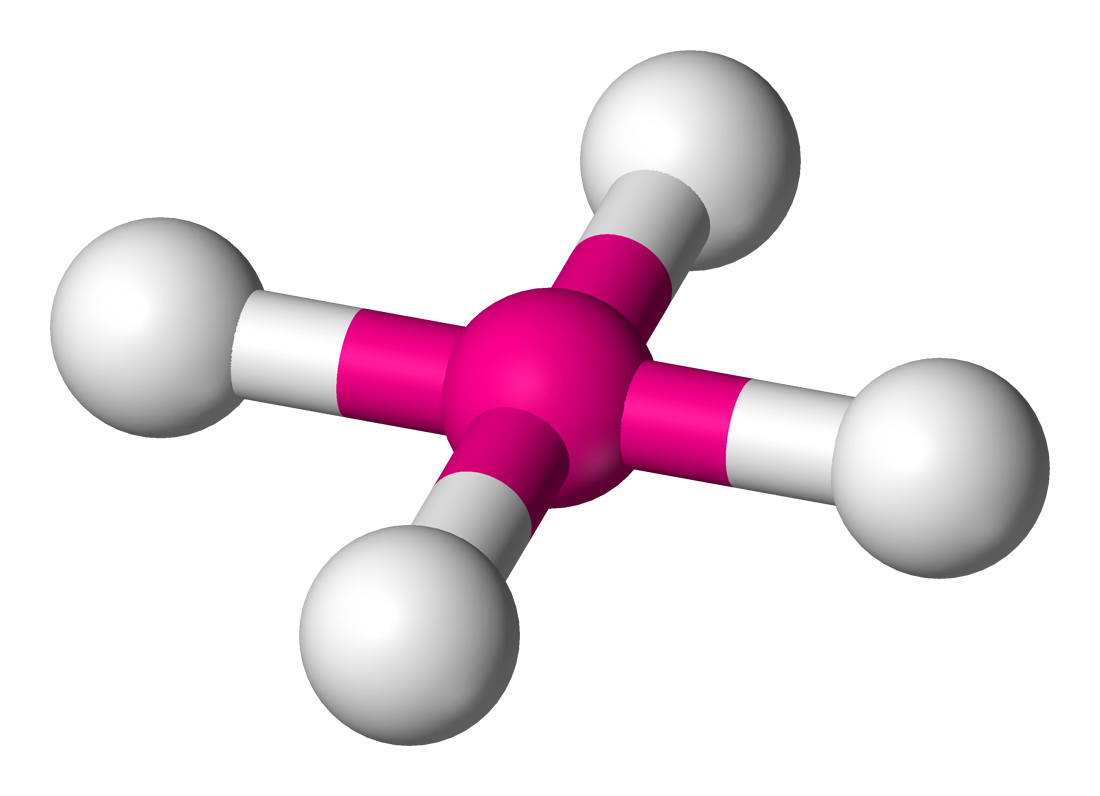
polarity:
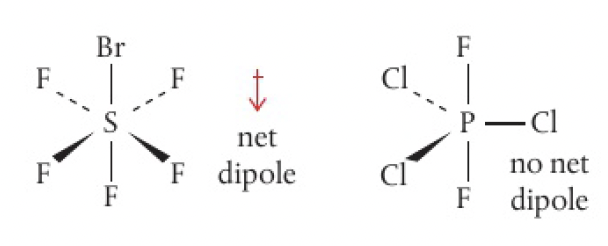
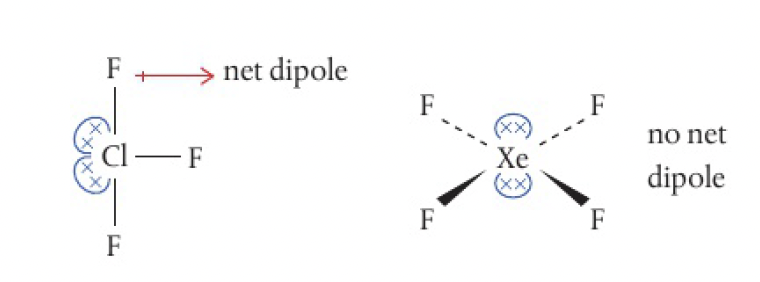
consider if there is a net dipole like before
challange questions
-
the position of bonding and non-bonding pairs of electrons in molecules or ions with five electron domains can be predicted by identifying the arrangement with the lowest number of lone pair-lone pair 90
angles followed by the lowest number of 90 angles between lone pairs and bonding pairs. can you use this approach to show why we observe see-saw, T-shaped, and linear geometries for
, , and seen in this section?