Structure 3.1.9 - the formation of variable oxidation states in transition elements can be explained by the fact that their successive ionisation energies are close in value
see 3.1.6 oxidation states
see 3.1.8 characteristic properties of transition elements (HL)
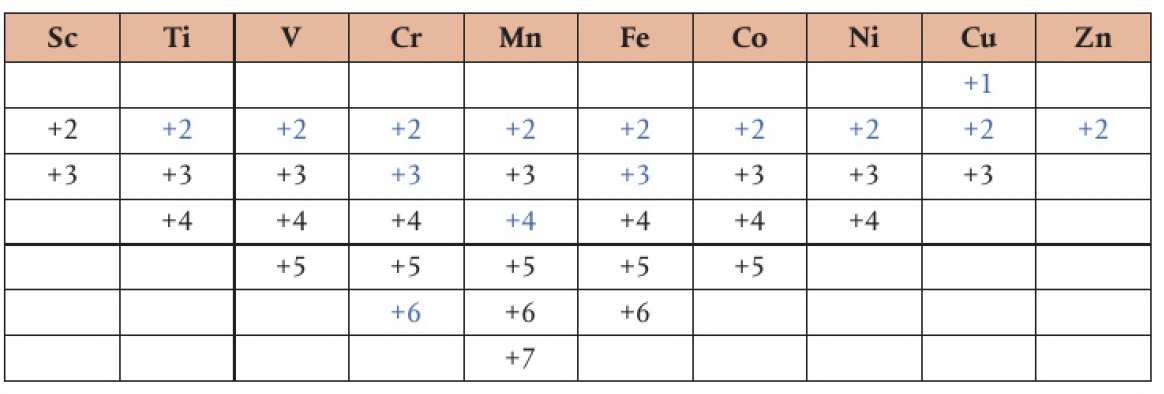
- all transition elements show +2 and +3 oxidation states
- oxidation states above +3 show covalent character
- ions of higher charge polarise negative ions and increase covalent character