Reactivity 2.2.6 - many reactions occur in a series of elementary steps. the slowest step determines the rate of the reaction
Reactivity 2.2.7 - energy profiles can be used to show the activation energy and transition state of the rate-determining step in a multistep reaction
Reactivity 2.2.8 - the molecularity of an elementary step is the number of reacting particles taking part in that step
reaction mechanism
- most reactions have a reaction mechanism which is a sequence of elementary steps that occur
- the mechanism is a theory about the sequence of events in progressing from reactants to products
Warning
while kinetic evidence can help to support a particular mechanism, it cannot be proven to be correct, only consistent with observed data
- the products of a single step in the mechanism are used in a subsequent step, existing only as reaction intermediates and not final products
- the sum of the individual steps in the mechanism must equal the overall equation for the reaction and intermediates on both sides cancel out
for example:
it has been shown that the mechanism involves the following elementary steps:
molecularity
molecularity is used in reference to an elementary step to indicate the number of reactant involved.
- unimolecular means there is 1 reactant particle involved
- bimolecular means that there are 2 reactant particles involved
- termolecular means that there are 3 reactant particles involved
termolecular reactions are rare because of the extremely low probability of more than two particles colliding at the same time with sufficient energy and in the correct orientation
the rate-determining step is the slowest step in the reaction mechanism:
- products of the reaction can only appear as fast as the products of this elementary step, so it determines the overall rate of the reaction
energy profile
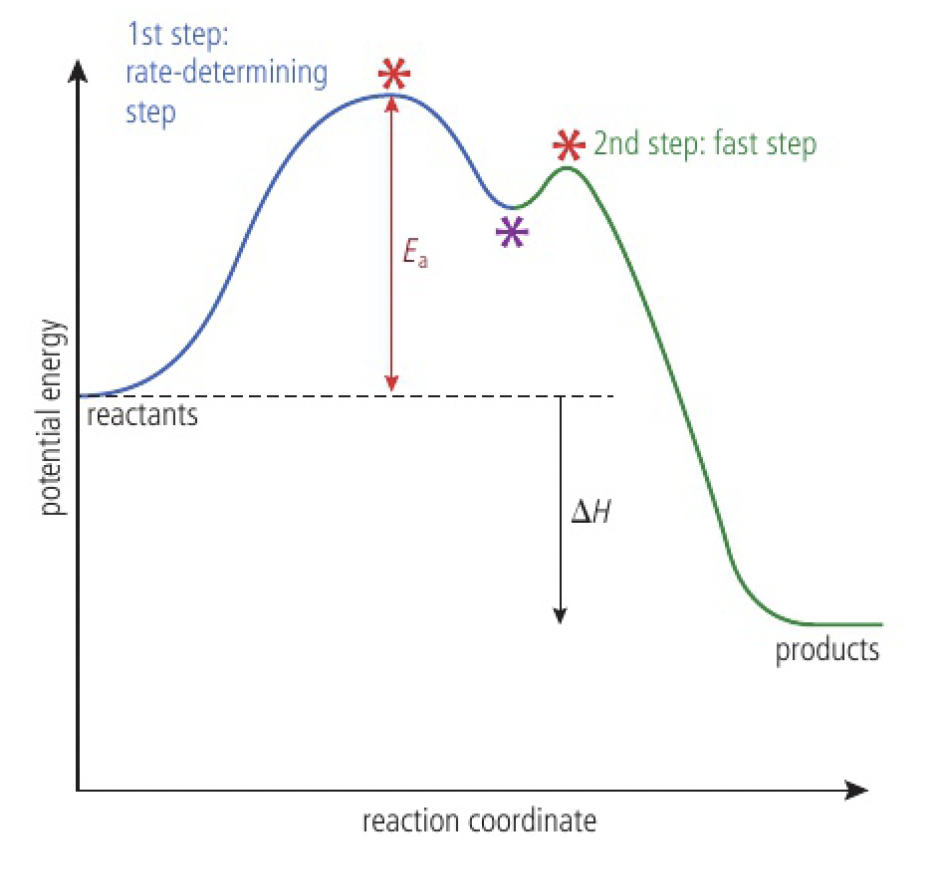
- the two maxima represent the transition states
- the minimum represents the intermediate species
- the activation energy for the overall equation is equal to the activation of the rate-determining step