chem modelsofbondingandstructure
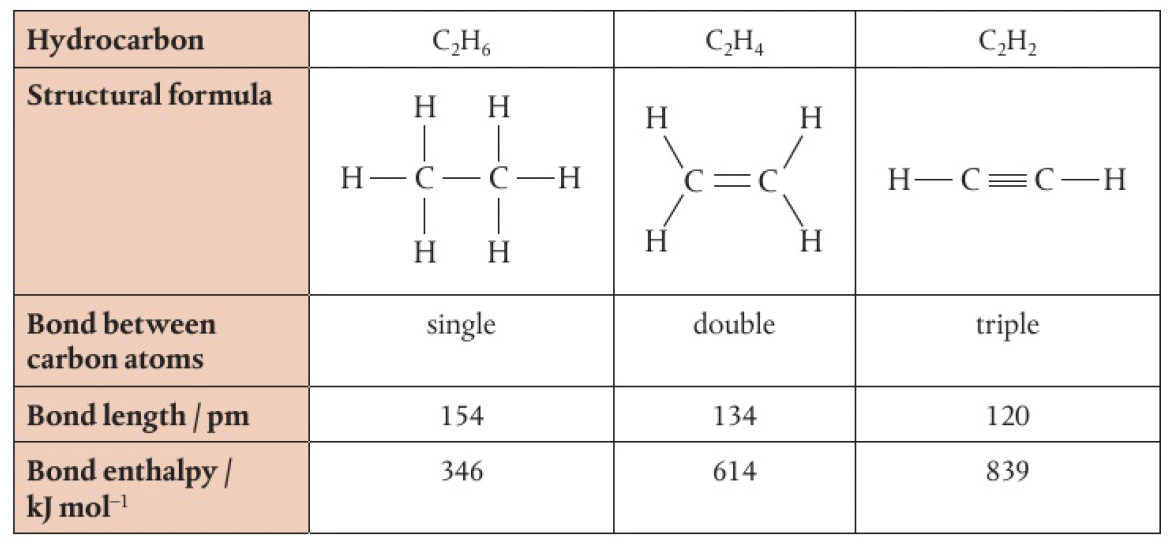
Structure 2.2.2 - single, double, and triple bonds involve one, two, and three shared pairs of electrons respectively
double bonds form when two electron pairs are shared
triple bonds form when three electron pairs are shared

multiple bonds contain unequal bonds. double bonds have one sigma bond and one pi bond, while triple bonds have one sigma bond and two pi bonds.
short bonds are strong bonds
- bond length is the measure of distance between two bonded nuclei
- bond strength is described in terms of bond enthalpy - the amount of energy required to break the bond
going down the group of halogens, it would be expected that bond length increases while bond enthalpy decreases due to the increasing atomic radius.
challenge questions
- fluorine,
is shown in brackets because a close look at its bond enthalpy data shows that it is an outlier in the trends described here. what explanation might account for this?
the bond length is so short that the lone pairs in the two atoms repel each other, weakening the bond