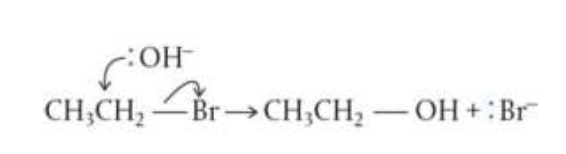chem whatarethemechanismsofchemicalchange
Reactivity 3.4.2 - in a nucleophilic substitution reaction, a nucleophile donates an electron pair to form a new bond, as another bond breaks producing a leaving group
halogenoalkanes
-
the halogen exerts a stronger pull on the shared electrons in the carbon-halogen bond (it is more electronegative)
-
the halogen gains a partial negative charge (
) -
the carbon gains a partial positive charge (
) - is said to be electron deficient
-
nucleophiles are attracted to the electron-deficient carbon
-
this leads to nucleophilic substitution reactions where the halogen is substituted
- the halogen atom can be substituted by a variety of nucleophiles
- halogenoalkanes are thus very useful intermediates in organic synthesis pathways
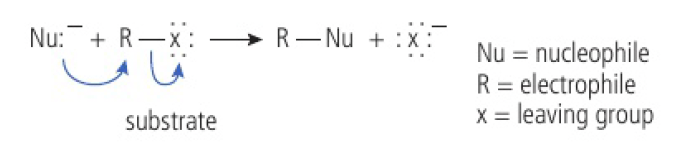
(the substrate here is
the curly arrows show the movement of electron pairs
-
for the nucleophile to form a coordination bond to the substrate molecule, another bond must break
-
a leaving group breaks away from the substrate
-
halogens are good leaving groups as they form relatively weak bonds with carbon
- the weakest bond generates the best leaving group
-
the higher electronegativity means that the bonded electrons are drawn towards the halogen atom
- thus, the carbon atom is electron deficient and susceptible to nucleophilic attack
NOTE
the bond that forms and the bond that breaks must involve the carbon atom that is bonded to the leaving group
if the nucleophile is a neutral species, the initial product formed from the substrate will be positively charged. however, this subsequently deprotonates and loses an
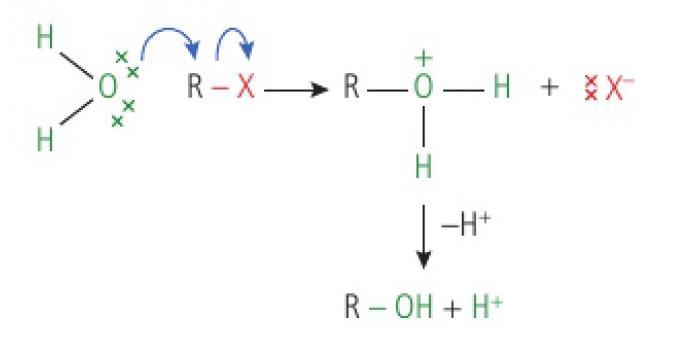
if the leaving group is a halide, it could combine with the