chem whatdriveschemicalreactions
Reactivity 1.4.2 - change in Gibbs energy,
- basing the direction of change on enthalpy changes leaves endothermic reactions unexplained
- basing the direction of change on entropy changes leaves reactions that occur with a decrease of entropy unexplained.
to consider the total entropy change of a reaction, the entropy change of the surroundings must also be considered
this reaction increases the entropy of the universe by transferring heat (energy) to the surroundings, leading to a more general dispersal of energy
exothermic reactions, with a negative
-
exothermic reactions are generally more common than endothermic reactions, not because of the decrease in energy of the system, but the associated increase in entropy of the surroundings
-
the impact of a transfer of heat to the surroundings on its entropy depends on the current state of dispersal
- if the surroundings have a high temperature, the addition of a little heat will make little difference
- if the surroundings are cold, same amount of heat causes a more dramatic change in entropy
calculating total entropy changes and understanding endothermic reactions
the second law of thermodynamics says that
substitute for
- endothermic reactions occur if the change in the entropy of the system can compensate for the negative entropy change of the surroundings produced as the heat is transferred from the surroundings to the system
- if
, then the reaction can occur spontaneously
Gibbs energy
this combination of entropy and enthalpy of a system gives a new function known as the Gibbs energy
-
a measure of how much energy that is free to do useful work rather than leaving the system as heat
-
energy left over that can do work after compensating for the entropy decrease in the system
-
spontaneous reactions have negative Gibbs energy changes because they can do useful work
the energy available to do work is
calculating
calculating
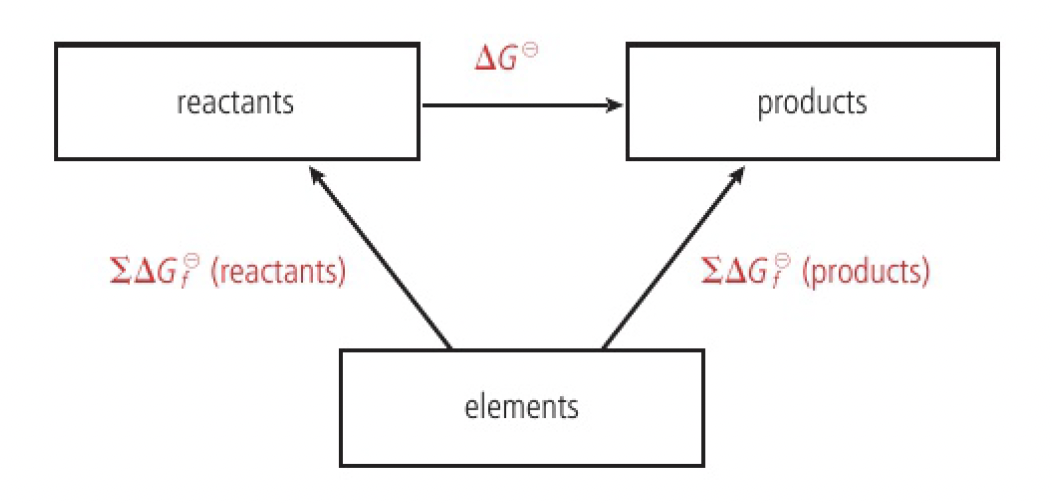
NOTE
just as
using
since the standard values of
as