chem modelsofparticulatenatureofmatter
Structure 1.3.6 - In an emission spectrum, the limit of convergence at a higher frequency responds to ionisation
Structure 1.3.7 - Successive ionisation energy data for an element give information about its electron configuration
the first ionisation energy of an element is the energy needed to remove one mole of electrons from the ground state of one mole of the gaseous atom
once removed, the electron is an infinite distance away from the nucleus and can be considered to be in the
the frequency at which convergence occurs can be obtained graphically. this can then be used to calculate the ionisation energies of atoms.
this is an example with the hydrogen atom:
the frequencies of the lines where the transitions involve the electron falling from the excited levels with
| excited energy level ( | frequency: | difference in frequency: |
|---|---|---|
| 2 | 24.66 | 4.57 |
| 3 | 29.23 | 1.60 |
| 4 | 30.83 | 0.74 |
| 5 | 31.57 | 0.4 |
| 6 | 31.97 | 0.24 |
| 7 | 32.21 | 0.16 |
| 8 | 32.37 |
a graph of
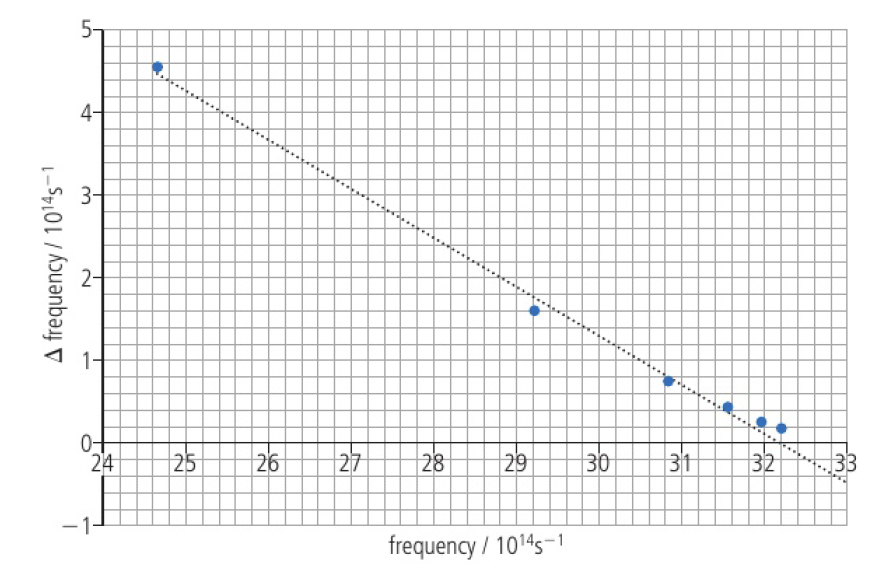
the line of best fit provides an approximate best-fit straight line with the intercept on the x-axis giving the frequency at which convergence occurs.
convergence occurs when
using the equation
thus, for one mole, the first ionisation of hydrogen is given by:
this is pretty close to the value of
successive ionisation energy data for an element gives information about its electron configuration
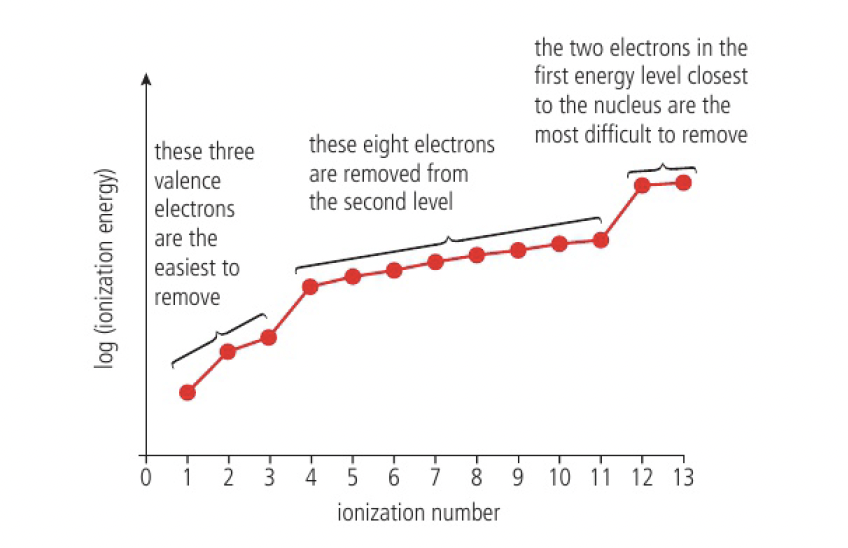
the figure above shows an increase in successive ionisation energies. due to:
- increasing attraction between increasingly positive ion and the negative electrons
- decreasing distance between the nucleus and the electron (each subsequent energy level is closer to the nucleus)
- less nuclear shielding with each successive removal
a closer look at the electrons in the n=2 energy level gives evidence for sublevels in the atom
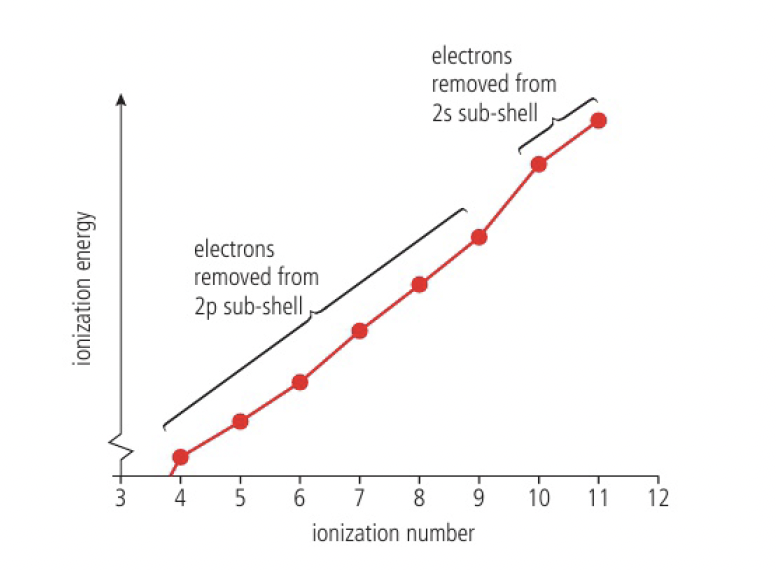
- the jump between the 9th and 10th ionisation energy shows that the 10th and 11th electron is more difficult to remove than would be expected from the pattern of previous electrons
- this suggests that the 2nd energy level is divided into 2 sublevels
also, take note of the slight difference between the 6th and 7th ionisation energies
- this is due to the particular stability of half-filled orbitals
- electrons in doubly-occupied orbitals repel each other, and so is easier to remove than an electron in a half-filled orbital
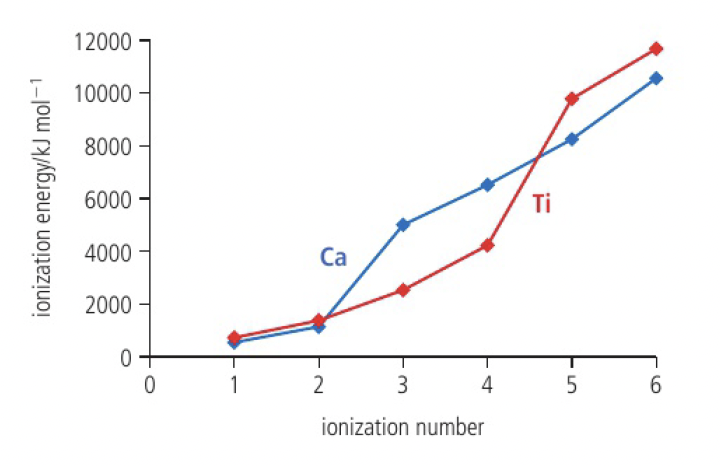
successive ionisation energies of the transition elements
one of the key features of transition metals is the wide range of oxidation states that the metals display in their compounds
- i.e. calcium only shows the +2 state, while titanium shows the +4, +3, and +2 states
the
however, for titanium, the increase in successive energies is more gradual as the 3d and 4s orbitals are close in energy (and so follows)
a large jump occurs between the 4th and 5th ionisation energies of titanium, as the inner 3p electron is removed, so titanium does not form the +5 state
trends in first ionisation energy across periods account for the existence of main energy levels and sublevels in atoms
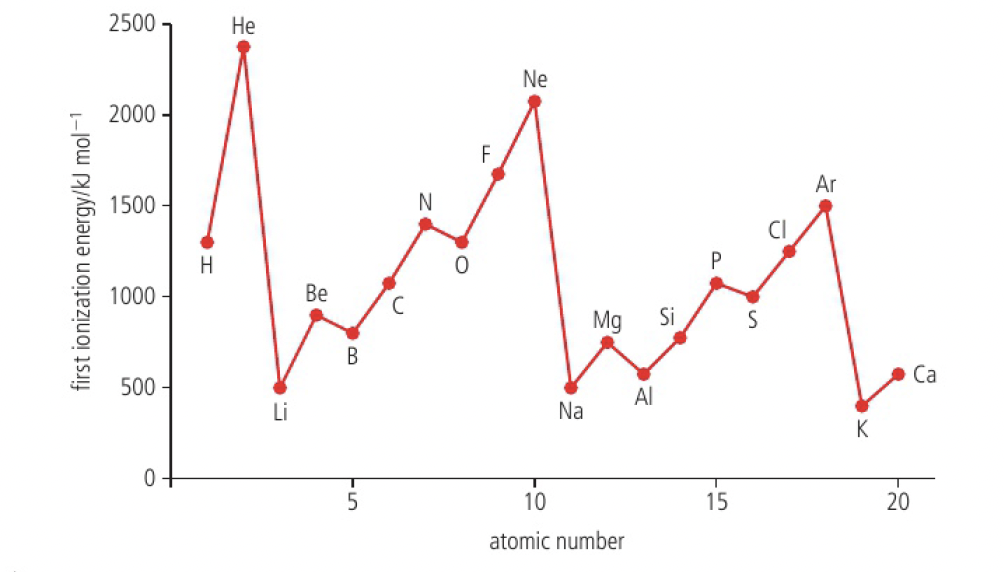
- ionisation energies generally increase from left to right across a period
- this is due to the increase of nuclear charge
- the electrons are removed from the same main energy level, but there is an increase in the force of electrostatic attraction
- ionisation energy decreases down a group as a new energy level, which is further from the nucleus, is occupied
- there are regular discontinuities in transition metals, which is explored in Structure 3.1
challenge questions
- A more accurate value for the convergence limit can also be found by plotting a graph of the frequencies of the lines against
. Determine the ionisation energy of hydrogen using this method and explain why this method is more accurate.
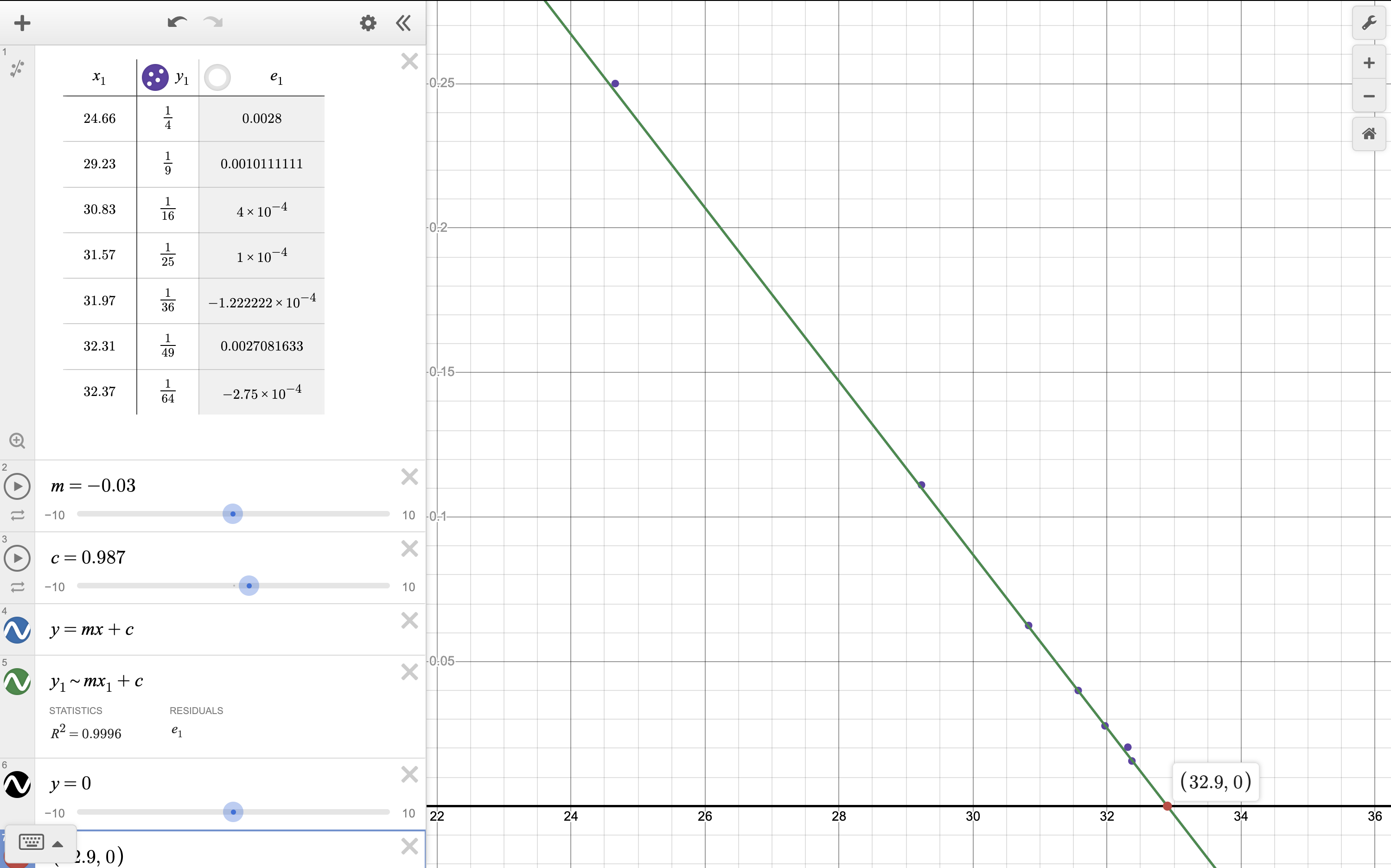
calculated 1313.12454kJ/mol. pretty close since the line of best fit was eyeballed
the points better fit a straight line than the other graph shown above
interesting
- Sodium lamps used for street lighting emit a distinctive orange light with a wavelength of 590 nm. Calculate the energy produced by one mole of excited sodium atoms when they give out orange light of this wavelength.