chem whatdriveschemicalreactions
Reactivity 1.3.4 - biofuels are produced from the biological fixation of carbon over a short period of time through photosynthesis
photosynthesis is redox reactions
chlorophyll, the green pigment in green plants, absorbs solar energy
biofuels are produced from the biological fixation of carbon over a short period of time
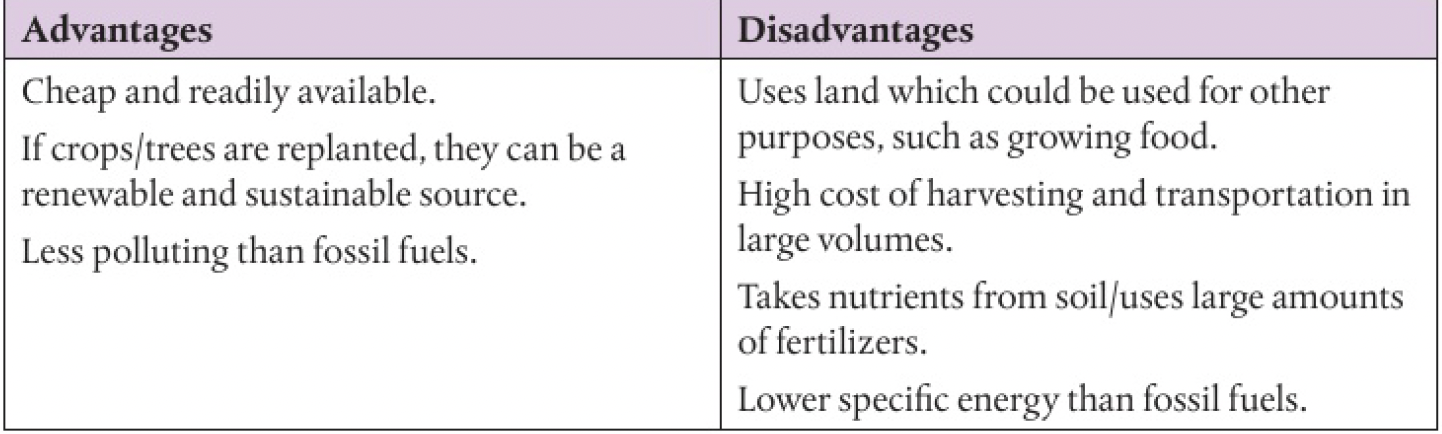
- renewable
- sustainable energy source
photosynthesis is not very efficient, so relatively little of the available solar energy is trapped
biofuels
- wood is mainly cellulose, a polymer of glucose molecules
- bioethanol can be made from biomass
- methanol can be produced by heating wood in the presence of steam and limited amounts of oxygen
pros of using bioethanol:
- renewable
- produces lower emissions
- decreases dependence on oil
cons of using bioethanol:
- it absorbs water (by forming hydrogen bonds with water in the atmosphere), separating from the hydrocarbon components in the fuel and causing corrosion
this fermentation process is typically done with plants high in starches and sugars, at around 37
gasohol is a mixture of 10% ethanol and 90% unleaded gasoline.
advantages and disadvantages of using biofuels
challenge questions
- one part of the Earth’s surface receives
of solar energy. green plants such as algae absorb of this energy.
a) determine the percentage of the sun’s energy absorbed green plants
b) suggest two reasons why the remainder of the Sun’s energy is not absorbed by green plants.
some wavelengths of radiation are not absorbed by chlorophyll. some radiation is reflected, some heats the surface of the Earth, plants don’t cover the entire surface of the earth
- a possible incomplete equation for the conversion of wood into hydrogen, carbon monoxide, and carbon dioxide is:
a) identify the oxidation number of all the elements in the equation
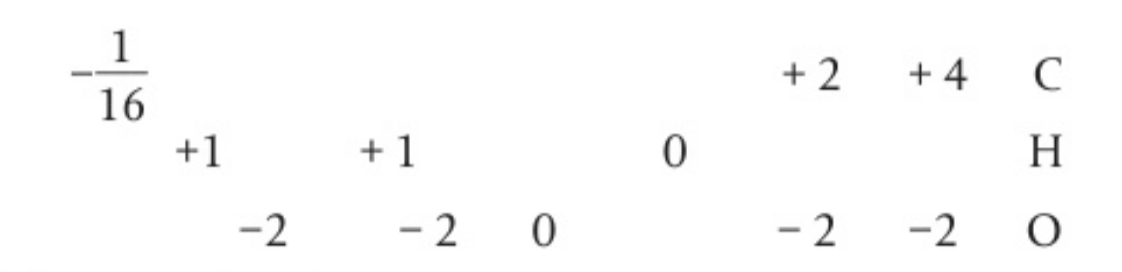
b) identify the elements which are oxidised and reduced in the reaction
c) deduce the value of
$$
\begin{align}
y+2z&=43 \
y+z&=32 \
z&=11 \
y&=21
\end{align}