chem whatarethemechanismsofchemicalchange
Reactivity 3.4.12 - the relative stability of carbocations in the addition reactions between hydrogen halides and unsymmetrical alkenes can be used to explain the reaction mechanism
addition reactions of unsymmetrical alkenes follow Markovnikov’s rule
Markovnikov’s rule:
-
the hydrogen will attach to the carbon that is already bonded to the greater number of hydrogens
-
the more electropositive part of the reacting species bonds to the least highly substituted carbon atom in the alkene
-
the addition of an unsymmetrical alkene has two potential products but only one is formed exclusively
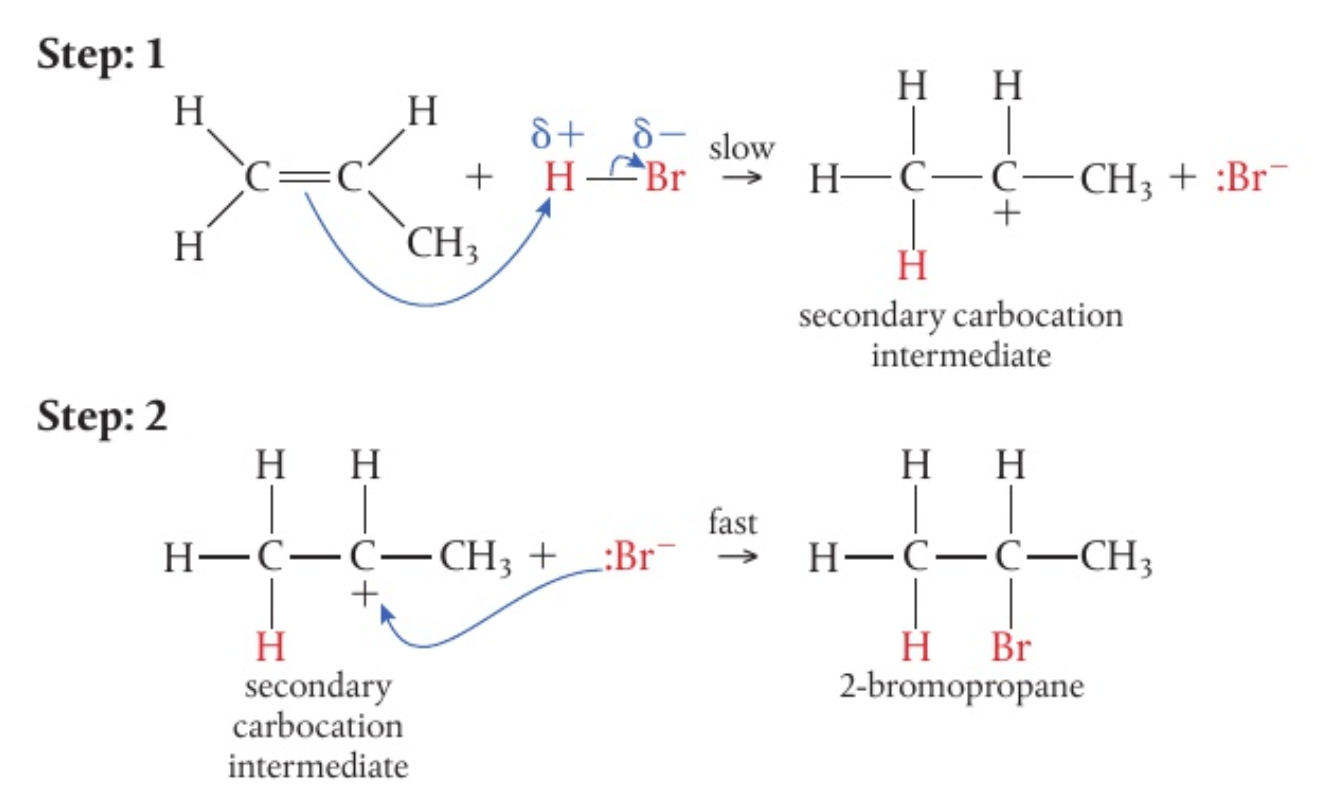
looking at the addition reaction ofwith , the specificity of this reaction can be explained
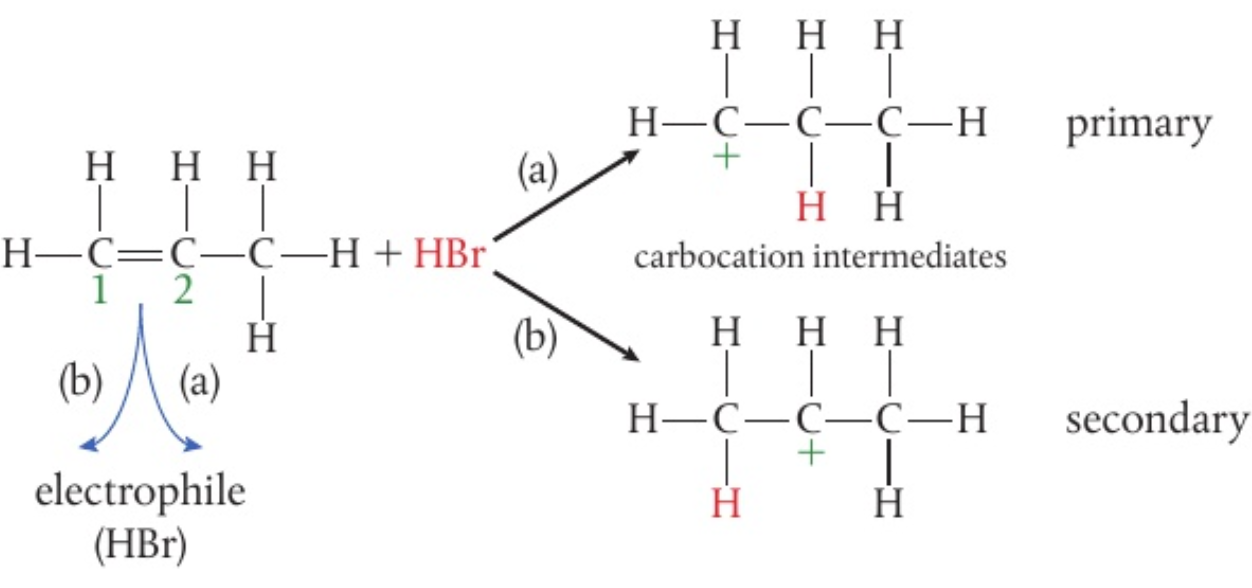
in the first step, the
- (a) a primary carbocation
- (b) a secondary carbocation
the secondary carbocation is more stable as:
- there are 2 positive inductive effects from the 2 alkyl groups around it compared to the primary carbocation
- the positive inductive effect pushes electron density away from the alkyl groups and towards the carbocation, lessening the density of the positive charge
thus, it is the more stable carbocation and more likely to persist and react with
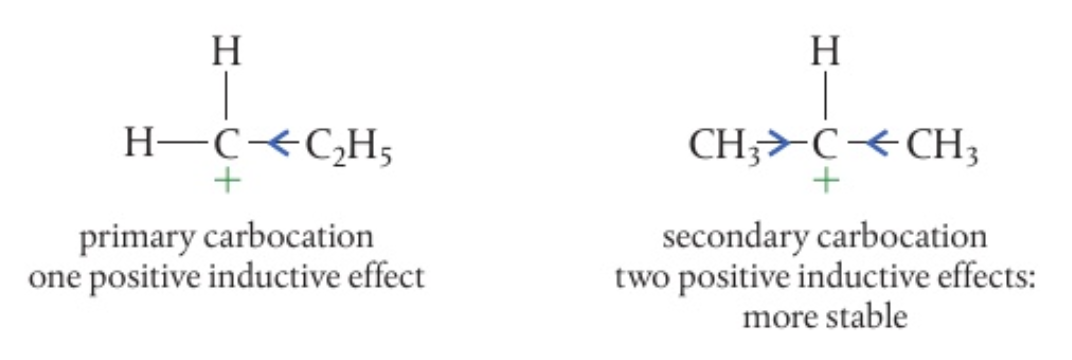
so then, 2-bromopropane will be the main product of the reaction
thus, the correct mechanism for the reaction is: