chem modelsofbondingandstructure
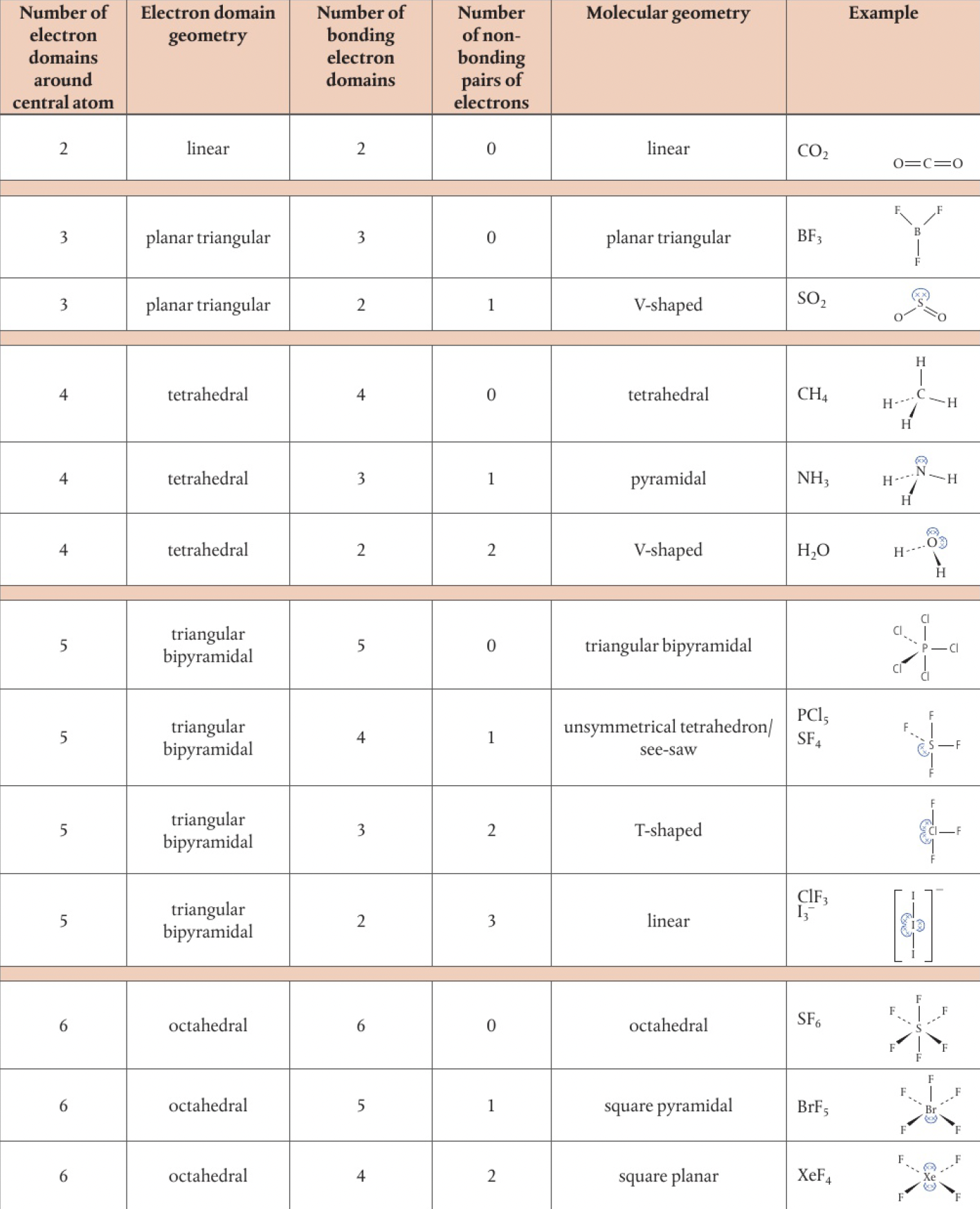
Structure 2.2.4 - the valence shell electron pair repulsion model enables the shapes of molecules to be predicted from the repulsion of electron domains around a central atom
an electron domain is the area in which electrons are most likely to be found, bonding and non-bonding
electron pairs are an over-simplification, since molecules often have multiple pairs of shared electrons. electron domain should be used, non-bonding, single, double, or triple pairs. the total number of electron domains around the central atom is what’s important
- the total number of electron domains around the central atom determines the geometrical arrangement of the electron domains
- the shape of the molecule is determined by the angles between the bonded atoms
- non-bonding pairs and multiple bonds cause more repulsion than a bonding pair for these reasons:
- lone pairs have a higher concentration of charge as they are not shared between two atoms
- multiple bonds have a higher concentration as they contain two or three pairs of electrons
as a result, molecules with lone pairs or multiple bonds on the central atom have some distortions in the structure that reduce the angle
two domains
- linear molecule
C#CC#Nthree domains
- triangular planar electron domain geometry
- if all 3 domains are bonding, then the molecular geometry will also be triangular planar
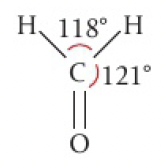
[H]B([H])([H])Cl[Al](Cl)(Cl)[H][C]([H])=Othere is some distortion in HCOH due to the increased repulsion of the double bond, so it should look more like
the Lewis formula of ozone,
[O]=[O]-[O]four domains
- tetrahedral electron domain geometry
- if all domains are bonding, the molecular geometry will be tetrahedral
- consider bonding pairs otherwise
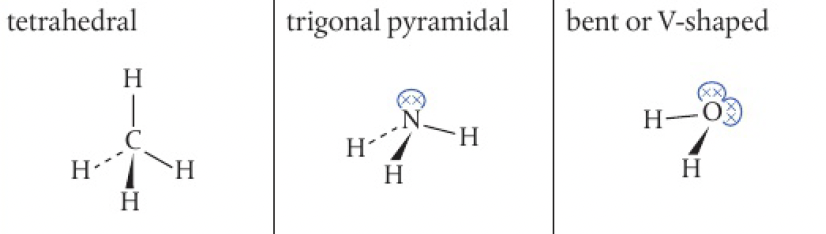
notice the greater reduction in bond angle in