chem whatdriveschemicalreactions
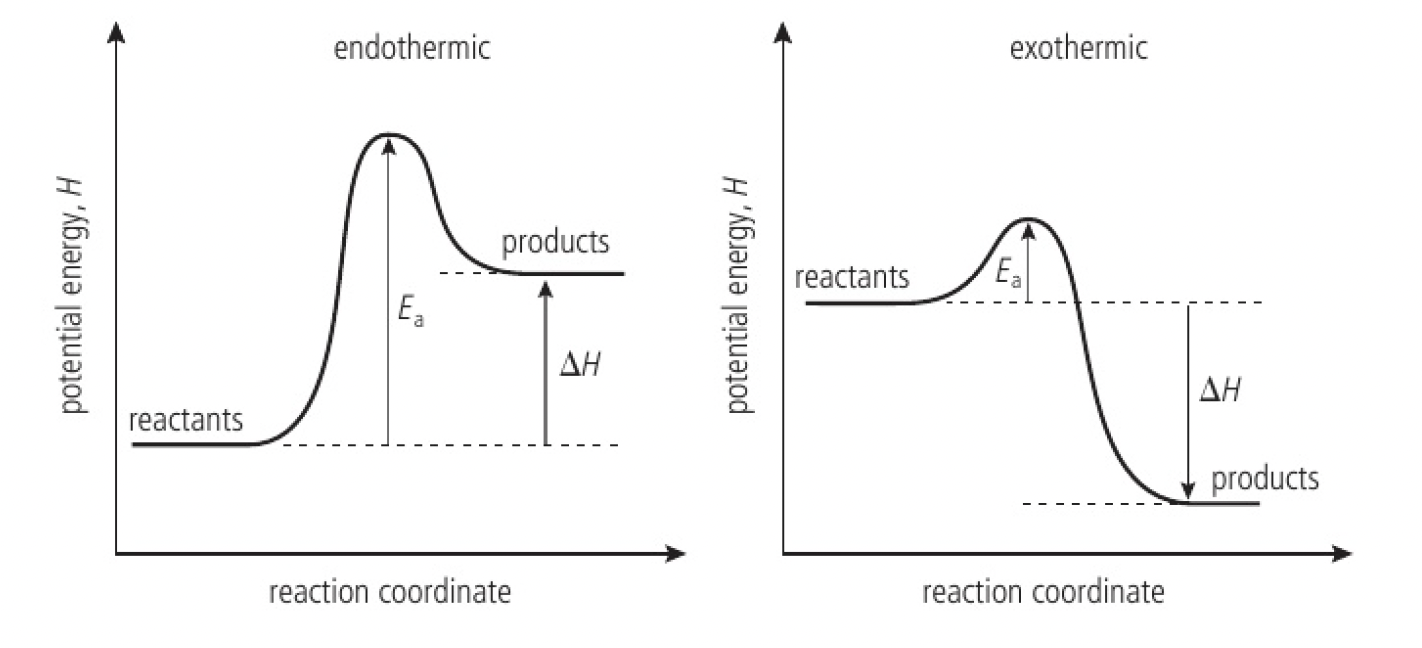
Reactivity 1.1.3 - the relative stability of reactants and products determines whether reactions are endothermic or exothermic
there is a natural direction for change:
-
in the direction of lower stored energy
-
in the direction of lower potential energy
-
reactions can be expected to occur if it leads to a reduction in potential energy, increased stability.
limitations:
- stability is a relative term, and hydrogen peroxide is stable with respect to its constituent elements, but unstable relative to its decomposition to water and oxygen.
- sometimes endothermic reactions do occur spontaneously
- using enthalpy change as a guide to change does not indicate the rate of reaction
some reactions do not occur at a noticeable rate as the reactants need to be given some energy to react:
- the minimum kinetic energy to react is known as the activation energy,
- this energy is needed as some bonds in the reactants must be broken before new bonds in the products can form