chem modelsofparticulatenatureofmatter
Structure 1.3.1 - Emission spectra are produced by atoms emitting photons when electrons in excited states return to lower energy levels.
Structure 1.3.2 - The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.
equations:
atoms of different elements give out light of a distinctive colour when an electric discharge is passed through a vapour of the element.
the flame test can be used to distinguish metals by spraying the dissolved ion of the metal through the flame of a bunsen burner
electromagnetic radiation (EMR) exists on a spectrum, as shown below
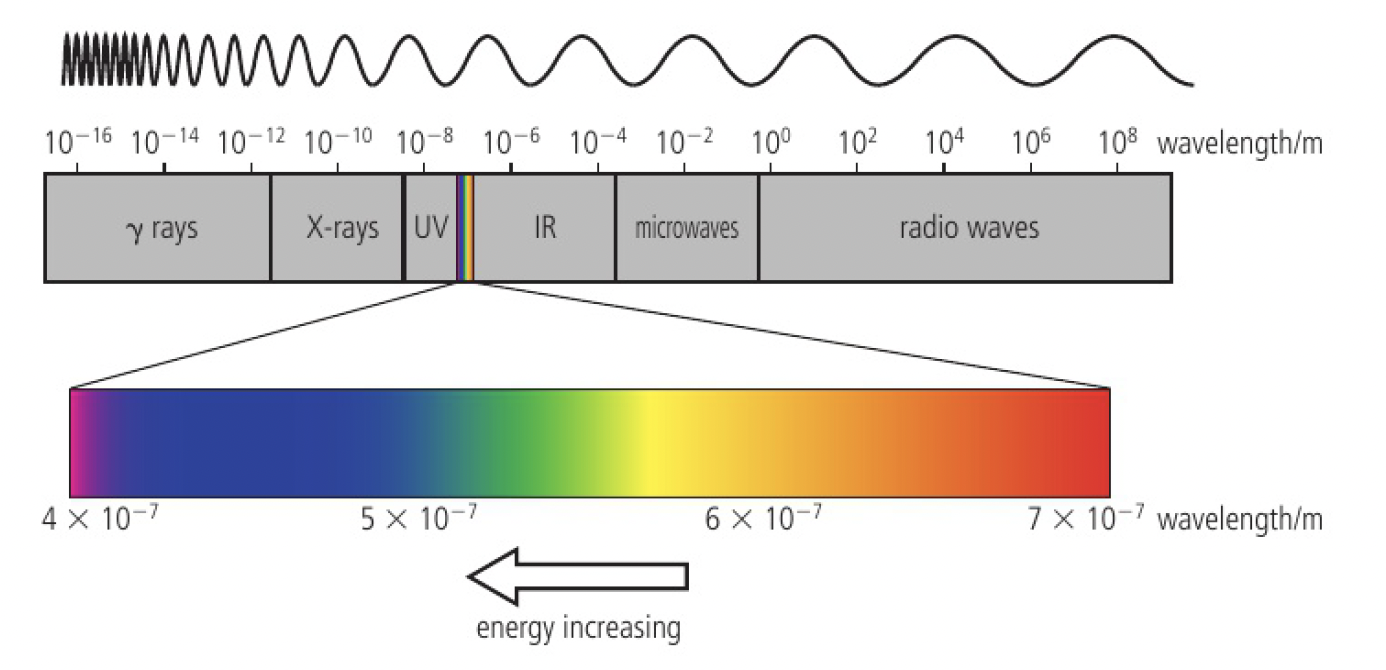
all EMR travels at the same speed,
the frequency
the shorter the wavelength, the higher the frequency
white light is a mixture of light waves of differing wavelengths. this can be seen when sunlight passes through a prism to produce a continuous spectrum
atoms also emit IR and UV, not just visible light
gases produce a characteristic emission line spectrum when heated to a high temperature or when a high voltage is applied.
the electrons are excited into a higher energy level that is unstable, so it soon falls back to the ground state. the energy given out when falling down is in the form of EMR. one packet of energy, a photon, is released for each transition downwards. the energy of the photon is proportional to the frequency of the radiation
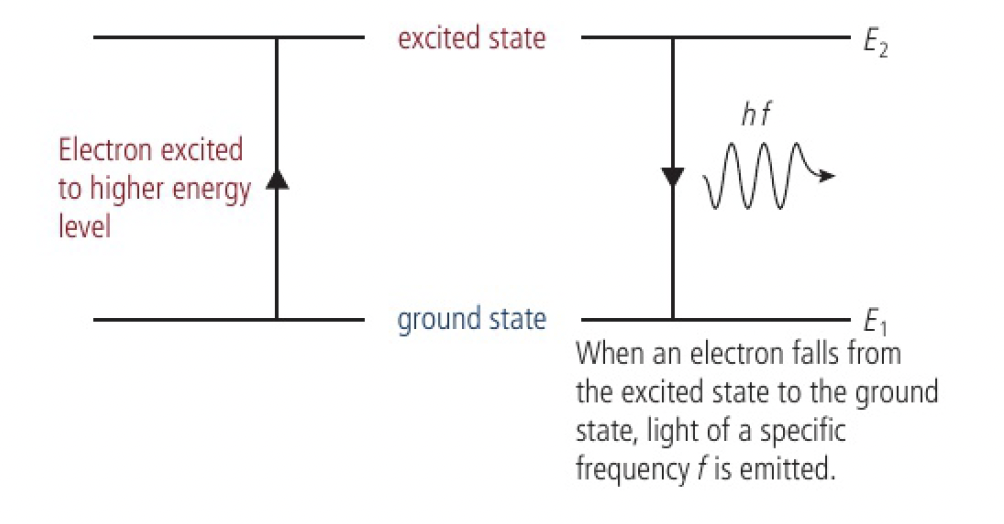
the energy of the photon of light emitted is equal to the energy change of the electron in the atom
atoms emit photons of certain energies, which give lines of certain frequencies as the electron can only occupy certain energy levels. the electron can only change its energy by discrete amounts - quantised. if the energy of the atom were not quantised, the emission spectrum would be continuous.
as different elements have different line spectra, they can be used to identify unknown elements
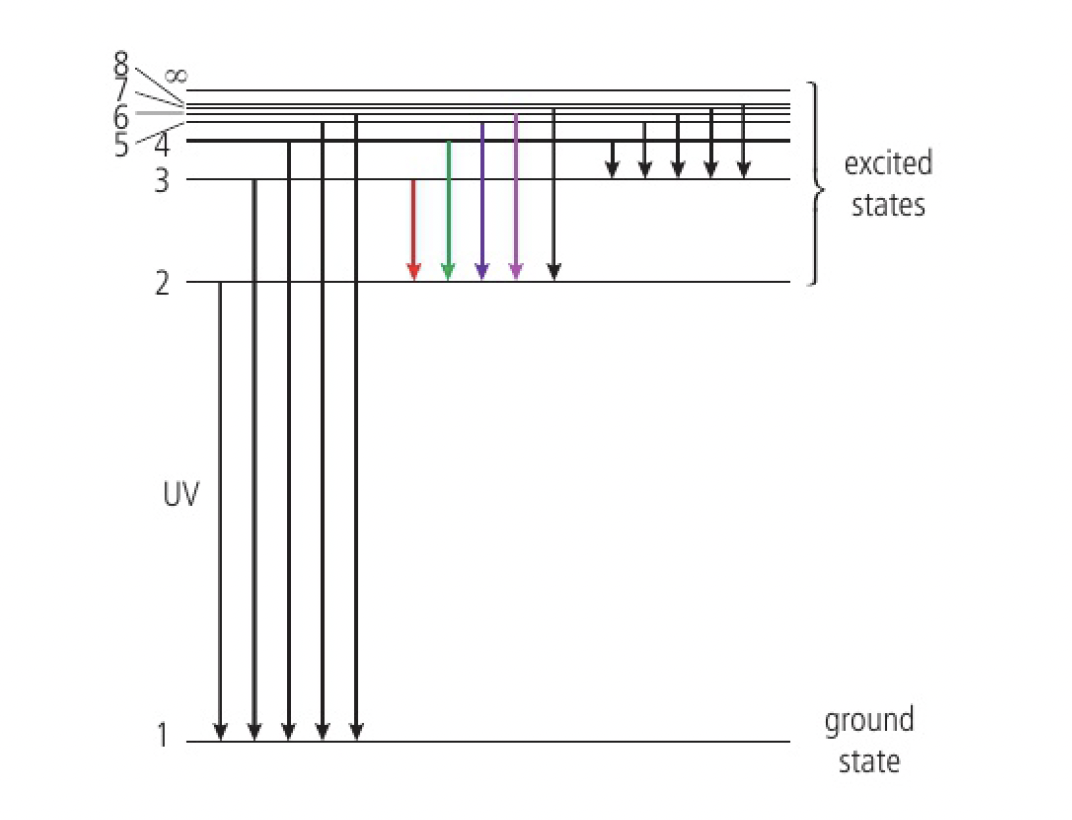
this is a diagram showing the transitions between energy levels of the hydrogen atom. the transitions to the 1st energy level correspond to the highest energy change, so give off UV EMR. visible light is produced when transitions to the 2nd energy level. to the third or higher energy levels, IR is produced, the lowest energy change.
the lines converge at higher energies because the energy levels inside the atoms are closer together.
when
challenge questions
-
One of the wavelengths in the emission spectrum of helium occurs at 558 nm.
Some energy levels of the helium atom are shown. The energies of the levels are given in joules. Identify the transition that produces the line at 588 nm.
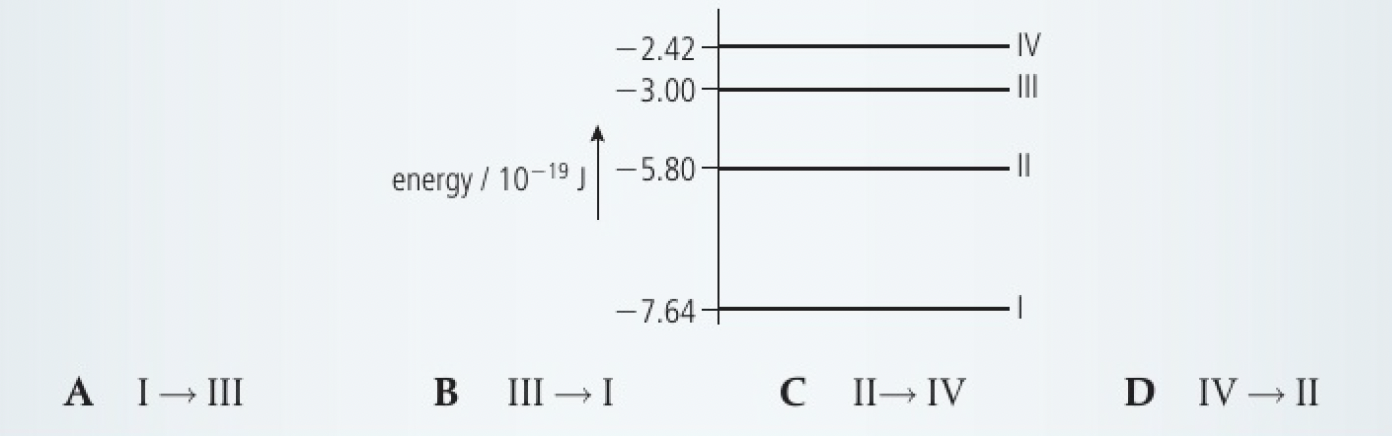
this best corresponds to the transition from IV