chem modelsofbondingandstructure
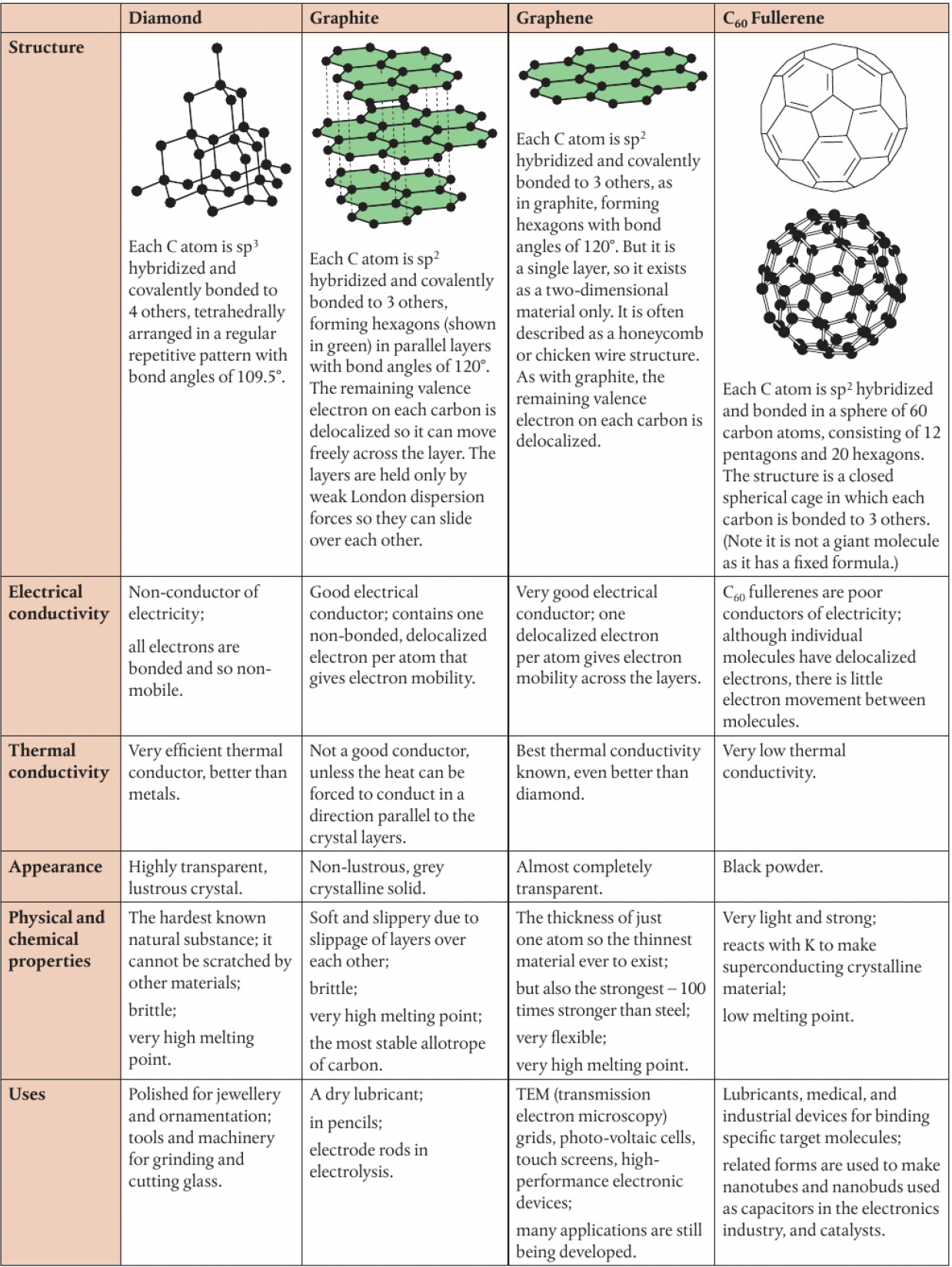
Structure 2.2.7 - carbon and silicon form covalent network structures
a covalent network structure is a covalent substance that exists in a crystalline lattice in which the atoms are linked together by covalent bonds - a single molecule with a regular repeating pattern of covalent bonds. (as opposed to other covalent substances which exist as discrete molecules, network structures have no finite size)
allotropes have different bonding and structural patterns of the same element in the same physical state. eg
carbon has several allotropes
challenge questions
- for the most part, good electrical conductors are also good thermal conductors, but this is not the case with diamond. can you think why this might be, and whether there are other substances that are not electrical conductors but good thermal conductors?
in diamond, each carbon atom is bonded to 4 other carbon atoms, leaving no charged particles that can move freely and thus no electrical conductivity.
the high thermal conductivity is due to its strong covalent bonds. when heated, the bonds become vibrationally excited, and as they are all connected, thermal energy can be readily transferred through the network
pure water does not conduct electricity but does conduct heat very well
- graphite is thermodynamically more stable than diamond. the reaction
is accompanied by a loss of energy, . But we commonly hear that ‘diamonds are forever’. are they?
the transition from diamond to graphite is energetically favourable, but the activation energy is too high at room temperature and pressure for the reaction to be observed.
silicon - another tetravalent element, also forms a giant lattice structure similar to diamond.
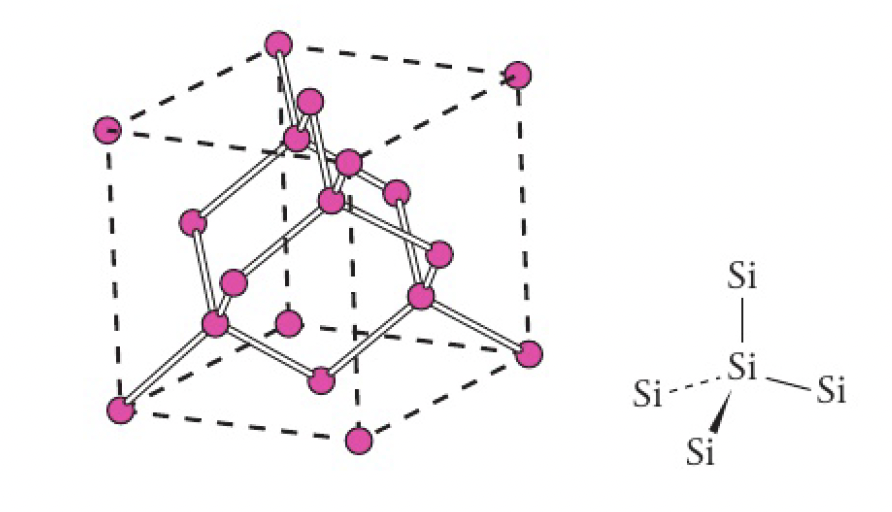
as the atoms are strongly held in tetrahedral positions that involve all four silicon valence electrons, the structure has the following properties:
- strong
- insoluble in water
- high melting point
- non-conductor of electricity
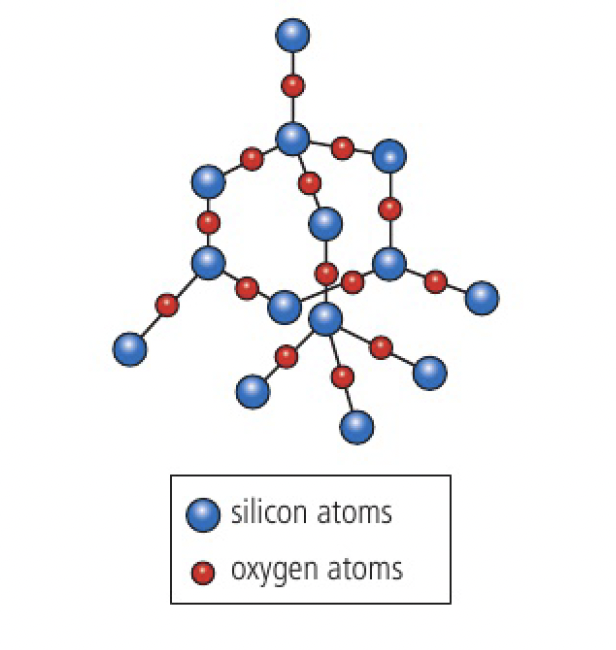
a notable difference between carbon and silicon is the tendency for carbon to form covalent bonds with other carbon atoms (catenation), whereas silicon forms bonds more commonly with oxygen. this is due to the greater bond strength of a