Structure 3.2.1 - organic compounds can be represented by different types of formulas. these include empirical, molecular, structural (full and condensed), stereochemical and skeletal
empirical formula is the simplest whole-number ratio of the atoms it contains. can be derived from percentage composition data from combustion analysis
molecular formula is the actual number of atoms of each element present in a compound
structural formula is a representation of the molecule showing how the atoms are bonded:
- full structural formula shows every bond and atom, usually with 60
, 90 and 180 angles - condensed structural formula omits bonds where they can be assumed, and groups atoms together
stereochemical formula shows the relative positions of atoms and groups around a central carbon in 3 dimensions
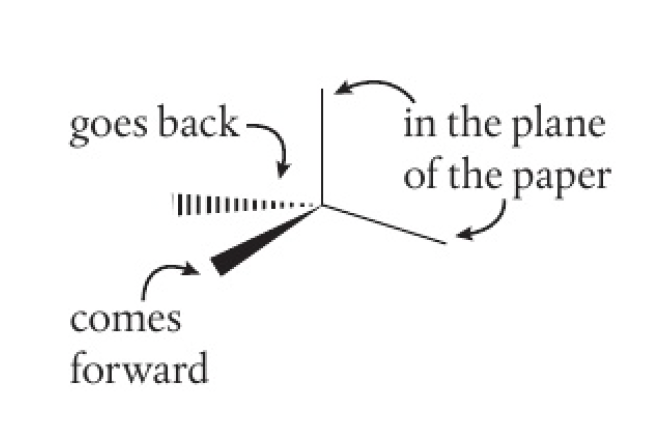
skeletal formula shows all bonds except
alkyls are just alkanes missing 1 hydrogen, which can be abbreviated to
for molecules with benzene rings, aromatic compounds, benzene can be shown as
c1ccccc1challenge questions
- based on experimental evidence, the bond length of a carbon-carbon single bond is 154 pm (1 pm =
m) and the radius of a carbon nucleus is m. if modelling kits were made to scale, how long would the plastic stick representing a single bond need to be if the carbon nucleus was represented as a plastic ball with a 0.5cm radius?
the plastic stick would need to be 57000 times longer than the nucleus, so
285m