chem modelsofbondingandstructure
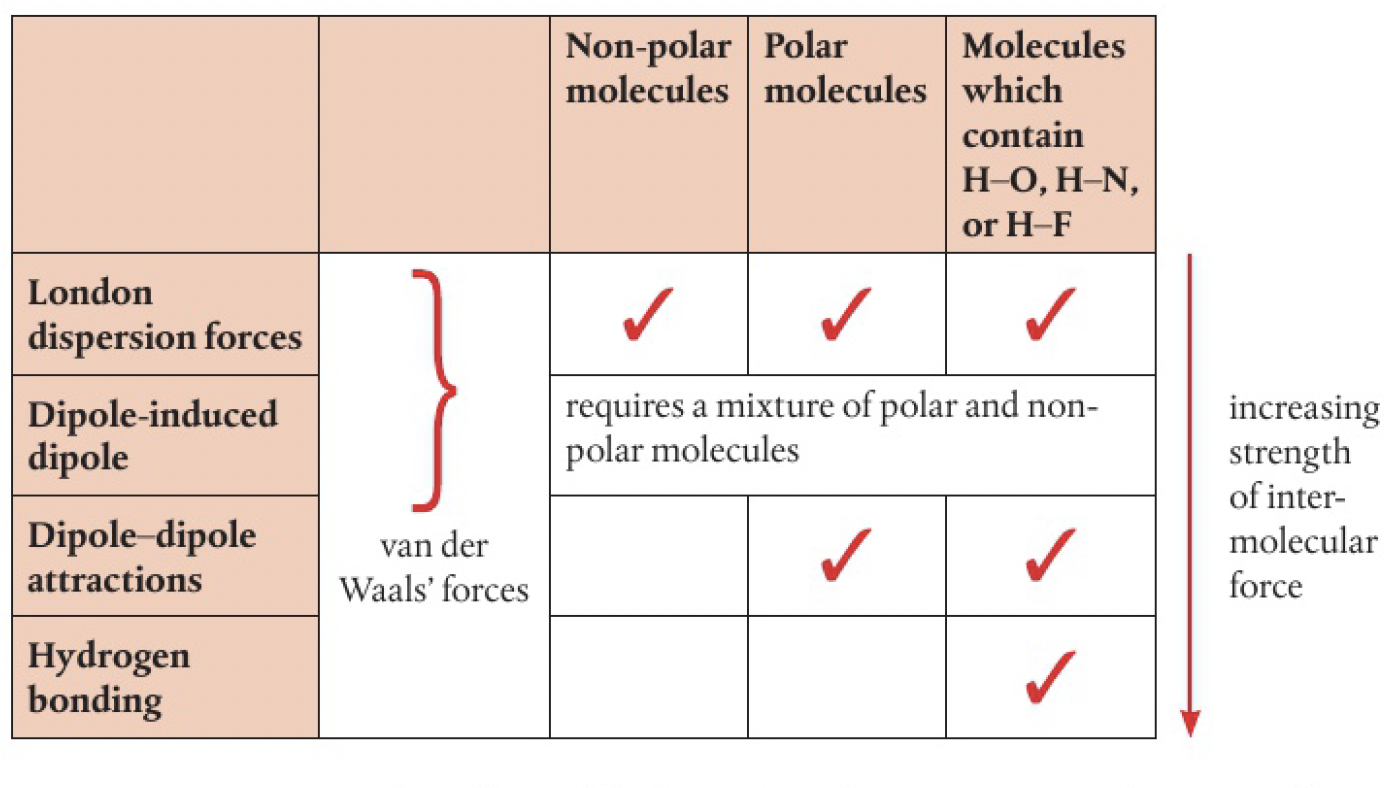
Structure 2.2.8 - the nature of the force that exists between molecules is determined by the size and polarity of the molecules. intermolecular forces include London (dispersion), dipole-induced dipole, dipole-dipole and hydrogen bonding.
van der Waal’s forces refer to all forces between molecules that do not involve electrostatic attractions between ions or bond formation
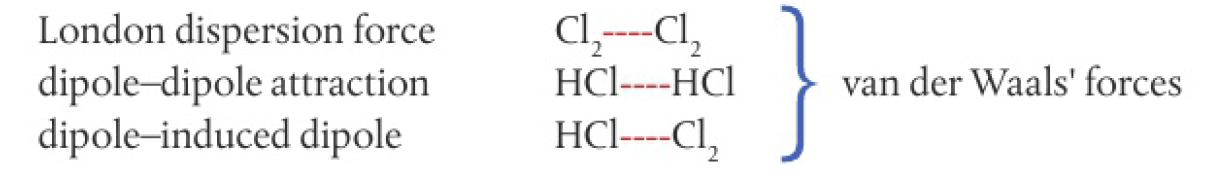
- hydrogen bonding, the strongest form of imf, is still 15-20 times weaker than covalent and ionic bonds
Structure 2.2.9 - given comparable molar mass, the relative strengths of intermolecular forces are generally: London forces < dipole-dipole forces < hydrogen bonding
the strength of intermolecular forces determines the physical properties of a substance, explaining volatility, solubility, and conductivity
imfs
london dispersion forces
electrons behave like mobile clouds of negative charge, where the density may be greater over one atom than the other. when this occurs, a weak dipole known as a ‘temporary’ or ‘instantaneous’ dipole will form. this will not last for more than an instant as the electron density is always changing, however, it may influence the electron distribution in the bond of a neighbouring molecule, causing an induced dipole.
this is the only force that exist between non-polar molecules. it exists in all molecules
with increasing molecular size, the strength of london dispersion forces increases. larger molecules have more electrons and larger electron clouds, thus, more susceptible to temporary fluctuations in electron distribution, having a greater probability of forming more temporary or instantaneous dipoles.
dipole-dipole attraction
polar molecules have a permanent separation of charge, where one end of the molecule
the strength varies depending on distance and relative orientation. dipole-dipole attractions can occur between any combination of polar molecules, and generally lead to the solubility of polar solutes in polar solvents.
dipole-induced dipole attraction
when a mixture contains both polar and non-polar molecules, the permanent dipole of a polar molecule can cause a temporary separation of charge on a non-polar molecule.
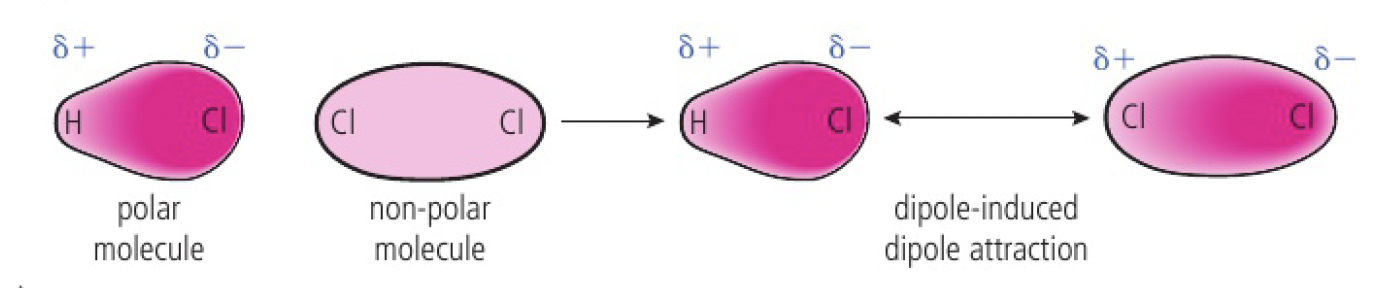
this acts in addition to london dispersion forces.
hydrogen bonding
occurs when a molecule contains hydrogen covalently bonded to
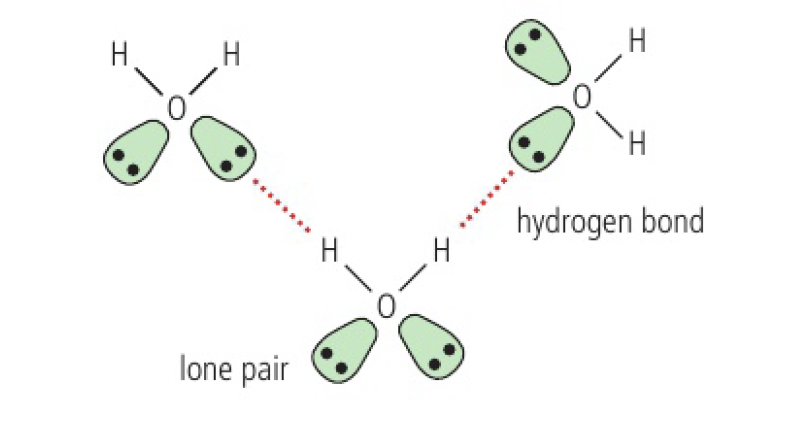
water
each
in ice, each
hydrogen bonding can also occur within large molecules
properties
general summary:
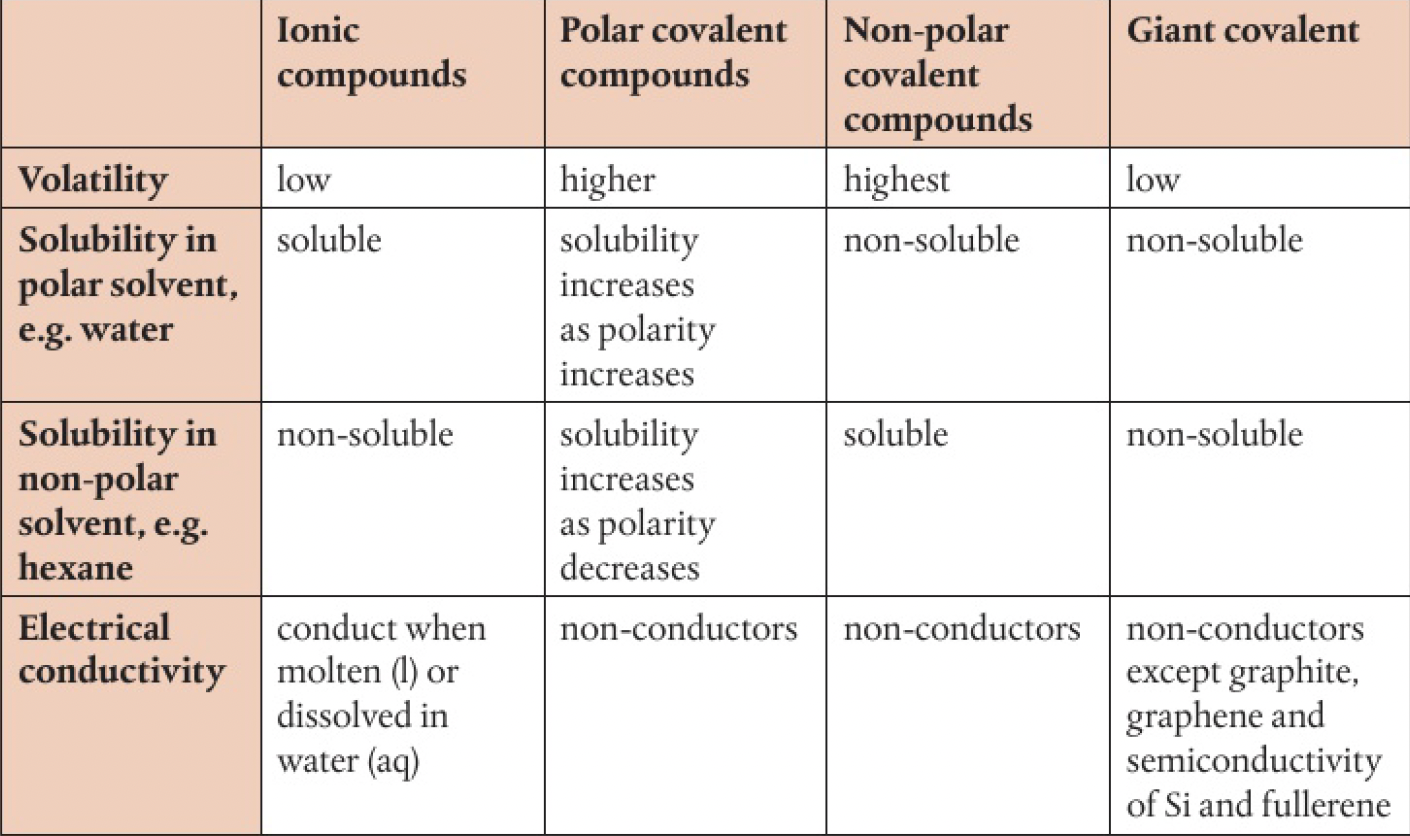
melting and boiling points
changing state by melting or boiling involves separating particles by overcoming the imfs. thus, the stronger the interparticle forces, the more energy required.
volatility
volatility describes the tendency of a substance to vaporise. a substance with stronger imfs will have a lower tendency to vaporise.
solubility
non-polar substances are generally able to dissolve in non-polar solvents by the formation of london dispersion forces.
polar compounds are generally soluble in water, interacting through dipole interactions and hydrogen bonding.
the solubility of polar compounds is reduced in larger molecules where the polar bond is only a small part of the total structure. the non-polar parts are unable to associate with water.
polar substances have low solubility in non-polar solvents since they will remain held to each other by dipole-dipole attractions.
giant molecular substances are generally insoluble in all solvents, since too much energy is needed to break the strong covalent bonds.
electrical conductivity
covalent compounds do not contain ions, so do not conduct electricity in the solid or liquid state. some polar covalent molecules (with ionic character) such as
challenge questions
- if you were given two solutions and told one contained a polar substance and the other a non-polar substance, suggest experiments that might enable you to identify which is which.
run from a burette with the stream next to a charged rod. the substance that deflects is polar due to the presence of a unbalanced electron distribution.
test each substance by trying to dissolve them in polar and non-polar solvents. see which dissolves in which.