chem modelsofbondingandstructure
Structure 2.4.3 - alloys are mixtures of a metal and other metals or non-metals. they have enhanced properties
see 2.3.1 and 2.3.2 metallic bond
see 1.1.1 elements, compounds and mixtures
formed by adding one metal element to another metal (or carbon) in the liquid state, so the different atoms mix. when solidified, the ions of different metals are scattered through the lattice uniformly (homogeneously).
this is possible because of the non-directional nature of the delocalised electrons and the lattice is able to accomodate ions of different size
alloys are often stronger, more chemically stable, more resistant to corrosion as the regular arrangement of atoms in a pure metal is interrupted by the presence of different cations, making it more difficult for atoms to slip over each other.
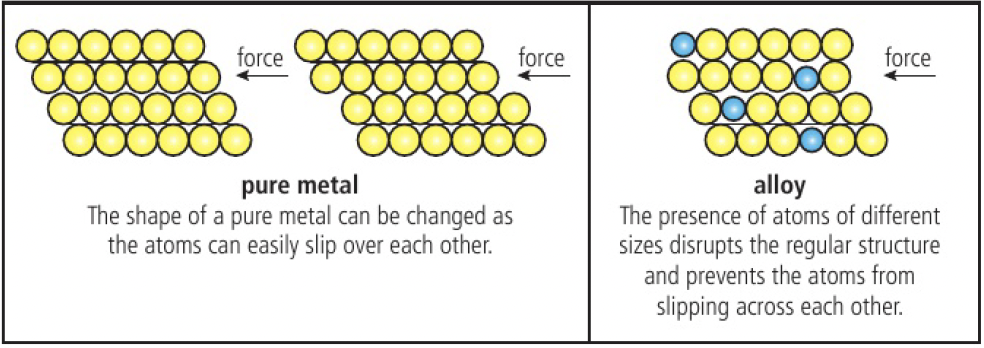
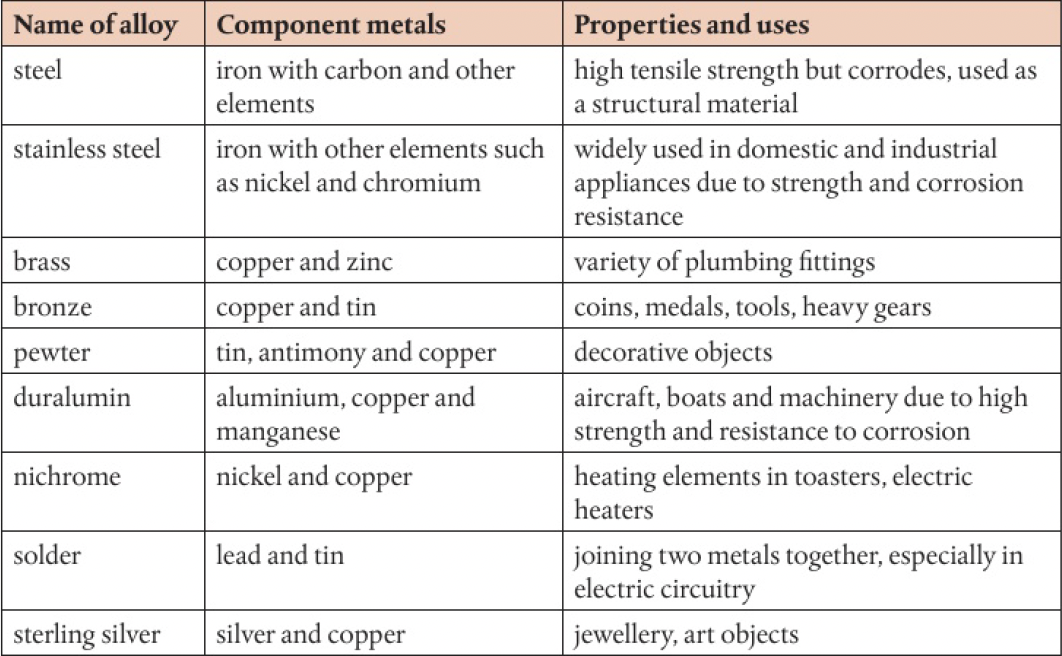
alloys have no fixed composition, so cannot be represented by a chemical formula. alloys are formed by mixing liquid metals without chemical reaction, so the components retain their metallic properties
challenge questions
- the components of a mixture can usually be separated by physical means. what might be the challenges of trying to separate metals from an alloy?
to separate the metals in an alloy, it must be heated to the melting point of the component with the lower melting point, leaving a solid and liquid. this is not economically efficient
alloys can also be separated using acids