chem modelsofbondingandstructure
Structure 2.3.1 - a metallic bond is the electrostatic attraction between a lattice of cations and delocalised electrons
Structure 2.3.2 - the strength of a metallic bond depends on the charge of the ions and the radius of the metal ion
metallic character refers to how easily elements lose their valence electrons
in the elemental state, valence electrons are loosely held by the metal atoms’ nuclei, so they become delocalised and spread themselves evenly throughout the cation lattice. (cations now because the atoms have lost their valence electrons)
properties and explanation:
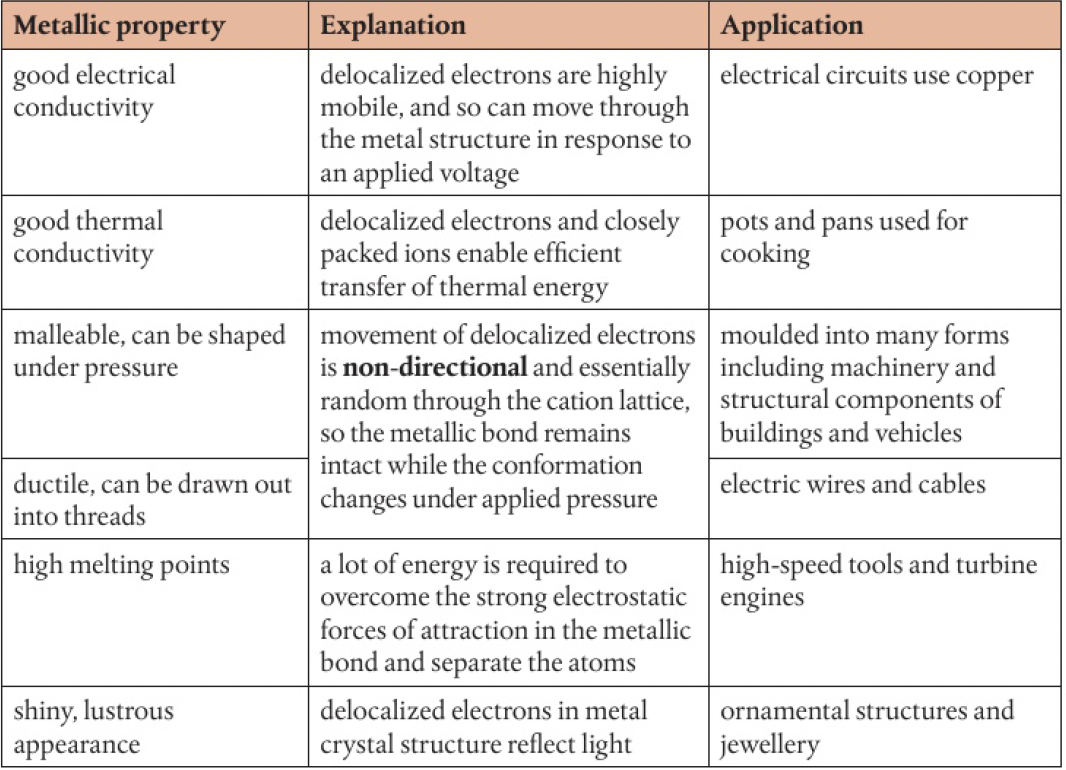
the strength of a metallic bond is determined by:
- the number of delocalised electrons
- the charge of the cation
- the radius of the cation
left to right across a period:
- increasing melting point (from more delocalised electrons)
- greater attraction between ions and delocalised electrons (from greater cation charge)
- lower reactivity (from lower bond strength)
down a group:
- decreasing melting point (from greater cation radius)
- weaker attraction between ions and delocalised electrons (from greater cation radius, more distance means weaker)
- higher reactivity (from greater cation radius, from weaker attraction)
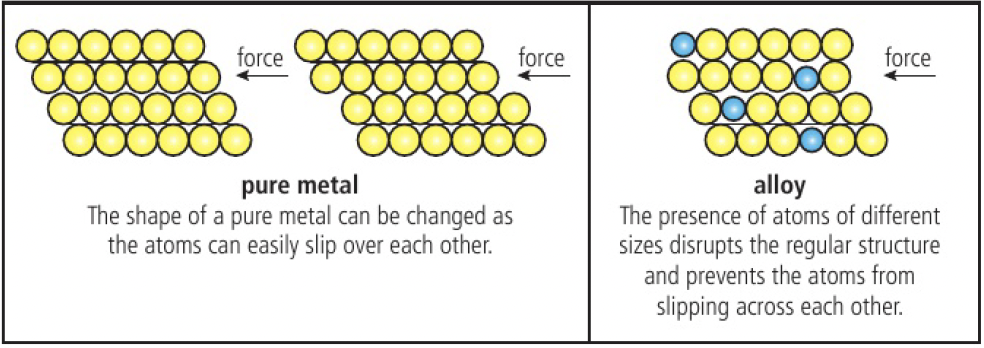
malleable because