chem modelsofbondingandstructure
Structure 2.2.15 sigma bonds (
pi bonds (
a bond can be thought of as when two atomic orbitals, each containing one electron, combine to form a new molecular orbital that is at a lower energy level
the bond axis is an imaginary line between two nuclei
the sigma (
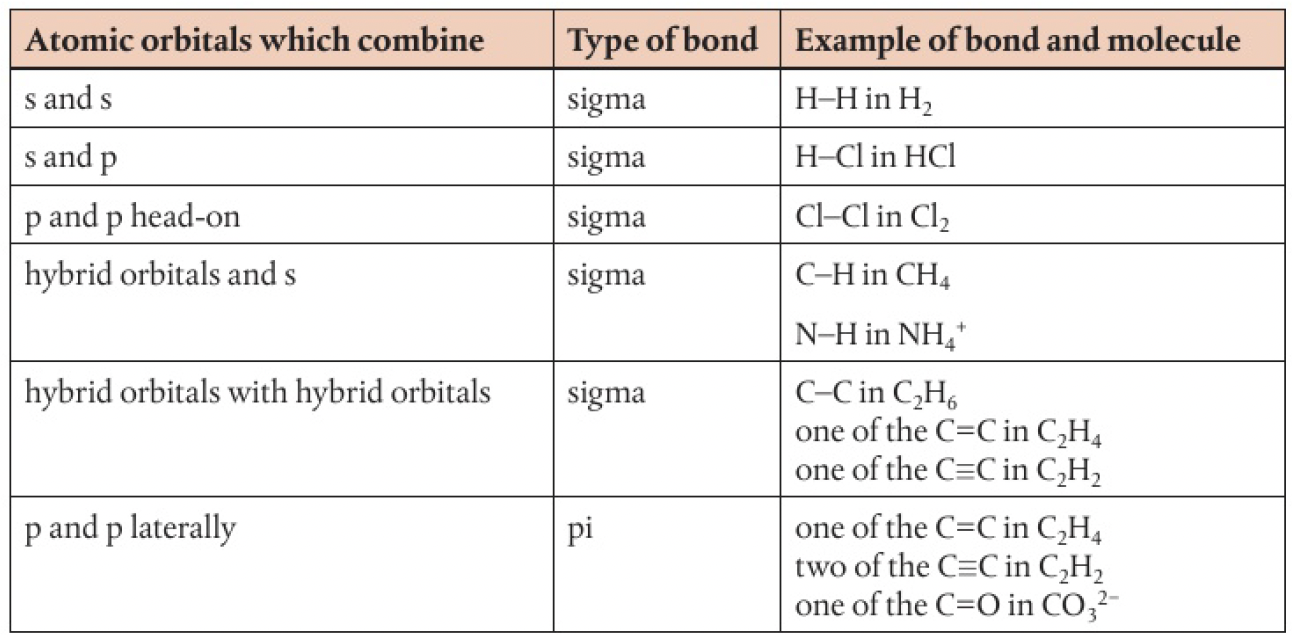
when two atomic orbitals combine head-on along the bond-axis, the bond is described as a sigma bond. electron density is concentrated between the nuclei of the bonded atoms.
this bond forms by the overlap of
- s orbitals
- p orbitals
- hybrid orbitals
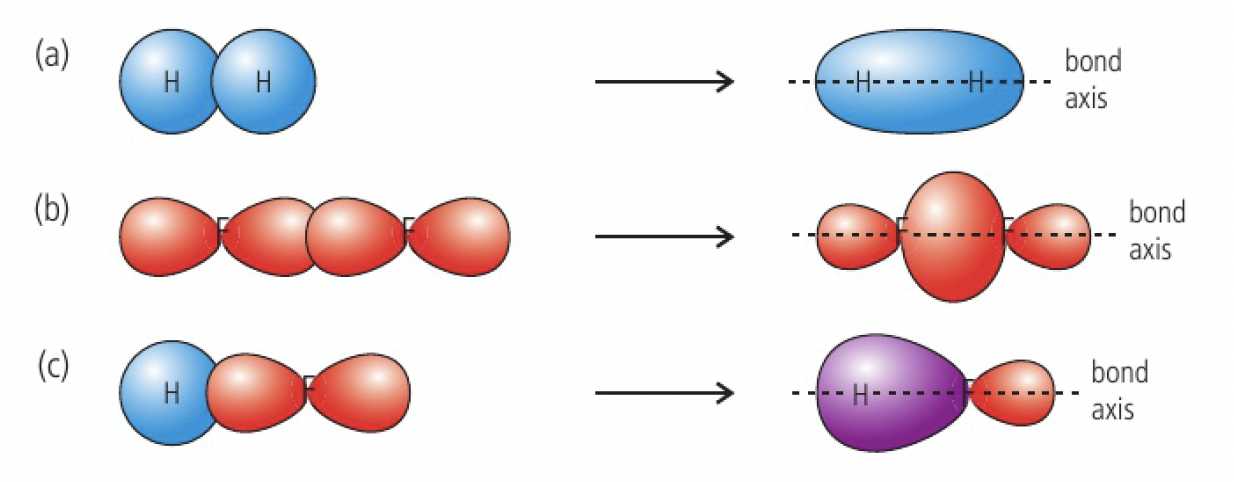
sigma bonds are stronger as the shared electron density is located closer to the two nuclei, resulting in a greater force of electrostatic attraction
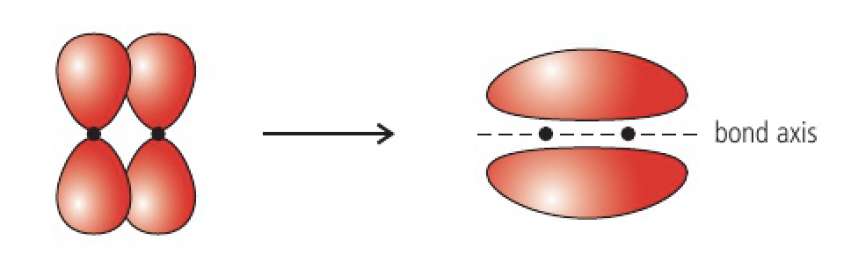
the pi (
when two p orbitals combine laterally, the electron density of the molecular orbital is concentrated in two regions, above and below the plane of the bond axis.
pi bonds are weaker than sigma bonds as the electron density is further away from the positive charge of the nucleus, so they break more easily during chemical reactions