Reactivity 2.2.3 - factors that influence the rate of a reaction include pressure, concentration, surface area, temperature and the presence of a catalyst
Reactivity 2.2.4 - activation energy,
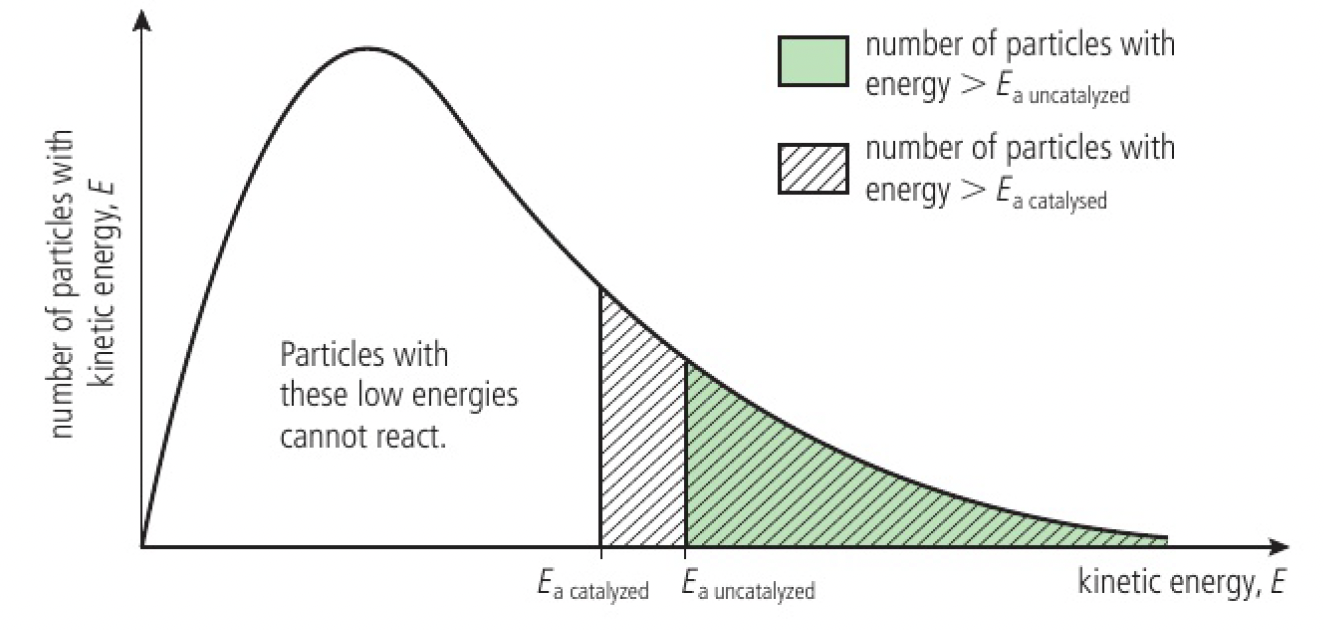
Reactivity 2.2.5 - catalysts increase the rate of reaction by providing an alternative reaction pathway with lower
temperature
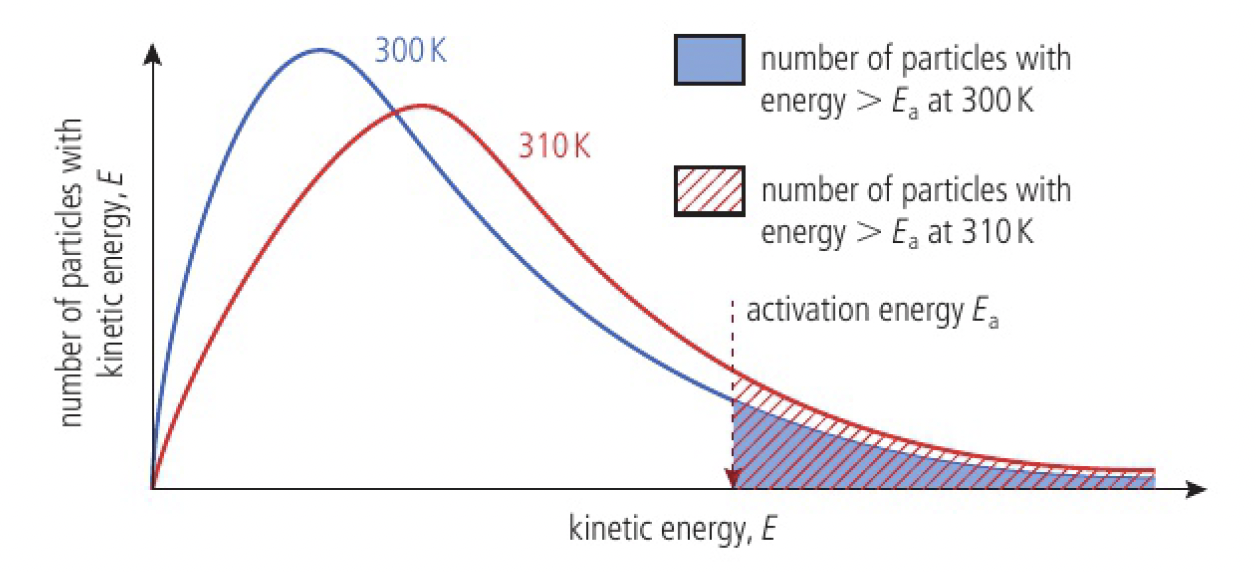
- increasing temperature causes an increase in the average kinetic energy
- leading to increase in collision frequency
- leading to increase in number of collisions with necessary activation energy (successful collisions)
concentration
- increasing concentration of reactants increases frequency of collisions between reactant particles and frequency of successful collisions
pressure
- increasing pressure in reactions involving gases increases the rate of reaction as it effectively increases the concentration of the gas and frequency of collisions
surface area
- increasing the surface area allows more contact and higher probability of collisions between reactants
- e.g. the reaction between
chips vs powder with hydrochloric acid.
catalysts
- a catalyst increases the rate of chemical reaction without undergoing chemical change
- most catalysts provide an alternative route for the reaction with a lower activation energy
- catalysts bring about an equal reduction in the activation energy of both the forward and revers reactions, so do not change the position of equilibrium or yield, but can increase the rate of reactions
- some reactions may proceed too slowly without a catalyst
- enzymes are biological catalysts
green chemistry:
- catalysis an important aspect of Green Chemistry, replacing stoichiometric reagents and can greatly enhance the selectivity of processes
- catalysts are effective in small quantities and can be reused, so do not contribute to chemical waste, increasing atom economy
- without catalysts, many reactions need to be conducted at very high temperatures
challenge questions
- catalysts can be categorised as ‘homogeneous’ or ‘heterogeneous’. a homogeneous catalyst is in the same phase as the reactants. for example, gaseous chlorine radicals catalyse the breakdown of ozone in the stratosphere. a heterogeneous catalyst, such as solid vanadium(V) oxide in the reaction between gaseous sulfur dioxide and oxygen, is in a different phase to the reactants. can you identify which type of catalyst might be more difficult to recover after a reaction and suggest a possible approach for doing so?
- homogeneous catalysts might be more difficult to recover after a reaction. there are several physical separation methods
- solvent extraction or distillation