chem modelsofbondingandstructure
Structure 2.1.1 - when metal atoms lose electrons, they form positive ions called cations
when non-metal atoms gains electrons, they form negative ions called anions
Structure 2.1.2 - the ionic bond is formed by electrostatic attractions between oppositely charged ions
to note:
- when ionic compounds are formed, the electrons first transfer, then the electrostatic attraction between the oppositely charged ions pulls the ions together.
- the attraction between ions increases with ionic charge.
- formation of increasingly negatively charged ions becomes more difficult due to increased electron-electron repulsion
- 1.3.6 and 1.3.7 ionisation energies (HL) too big jump in energy when electrons are removed from inner energy levels
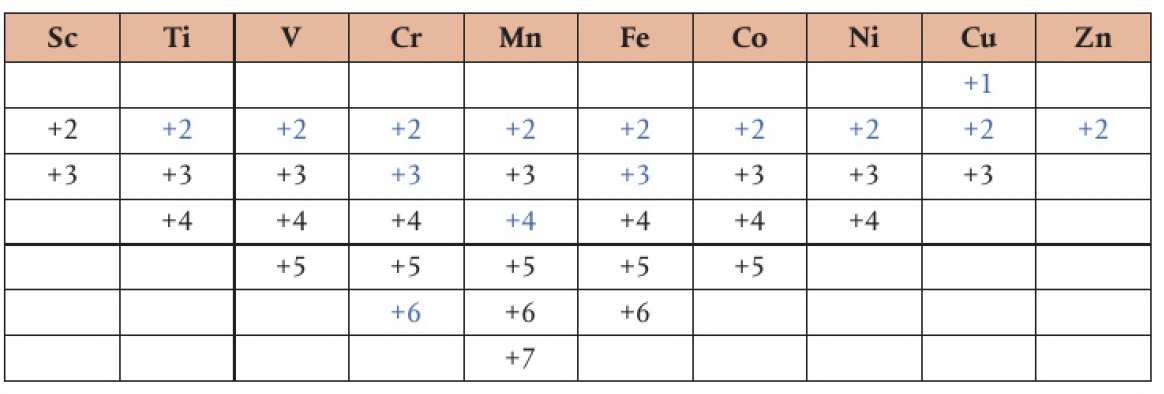
- charge is expressed as 3- or 3+, oxidation state is -3 or +3
and and - when naming ionic substances formed with metals with multiple oxidation states, include the oxidation number in the brackets
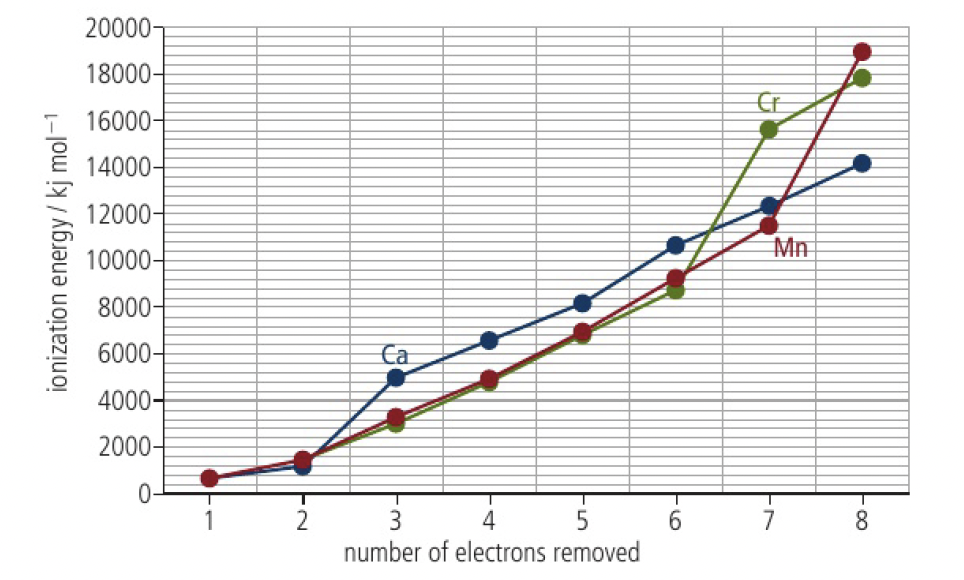
it is more correct to refer to oxidation states rather than ionic charge for such species of transition metals which can have a large range. the maximum oxidation state is reached when the linear pattern of successive ionisation energies ends, at 7 for
oxidation states for first row d block elements. common in blue
nuclear charge of the atom is given by the atomic number and increases by one between successive elements
the valence electrons, which determine the chemical properties, do not experience the full attraction of this charge due to shielding and repulsion by the inner electrons. they experience the effective nuclear charge.
metal atoms form positive ions as they have low ionisation energies. first ionisation energies increase across a period due to the increase in effective nuclear charge. as effective nuclear charge increases, the attraction between the nucleus and the valence electrons increases, making them more difficult to remove.
non-metal atoms form negative ions as they have high effective nuclear charges.
noble gases
the full outer shells of noble gases mean that they have very high ionisation energies. it also means that electrons added would experience an effective nuclear charge of zero, occupying an empty outer energy level. this explains the non-reactivity of the group
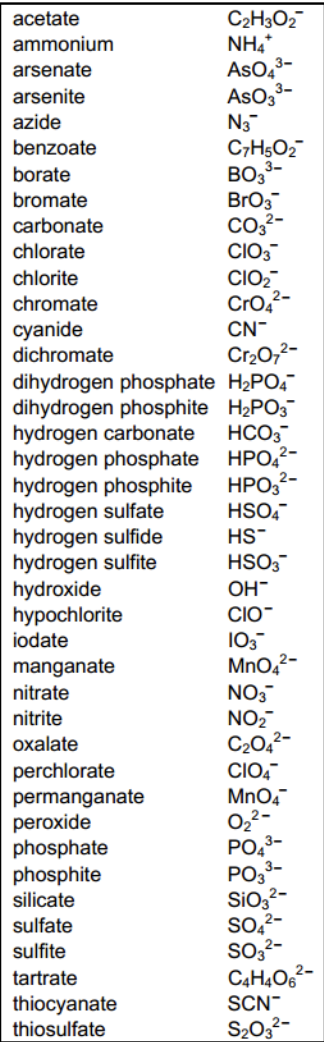
common polyatomic ions
note: bonding between atoms in polyatomic ions is covalent
challenge questions
- as shown in structure 1.3, although there is a generation increase in ionisation energies across a period, there are some regular discontinuities in this trend. the first ionisation energy of aluminium, for example, is lower than the first ionisation energy of magnesium. how can the calculation of effective nuclear charge be refined to explain these discontinuities?
“Our calculation does not include the shielding effect of electrons in a lower sublevel. The two 3s electrons in aluminium, for example, partially shield the 3p electron so it experiences a smaller effective nuclear charge than the +3 calculated using the simple model. The reduced effective nuclear charge makes it easier to remove the 3p electron from aluminium than to remove one of the 3s electrons in magnesium.”
- what evidence based on simple observations supports the existence of ions?
electrolysis of salts - NaCl, production of