chem whatarethemechanismsofchemicalchange
Reactivity 3.1.13 - pH curves of different combinations of strong and weak monoprotic acids and bases have characteristic shapes and features
this takes into consideration the different combinations of strong and weak acids and bases.
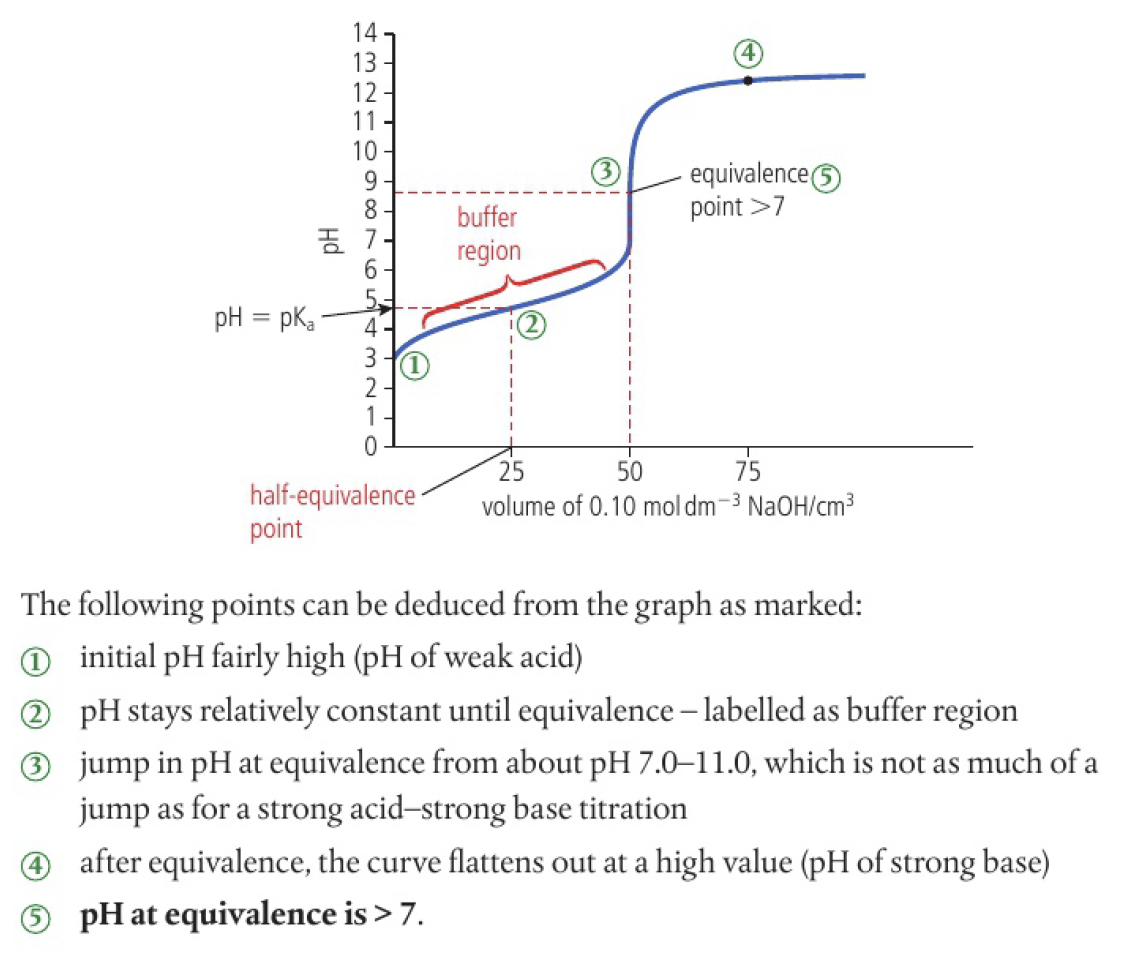
- the point labeled
is the half-equivalence point, where exactly half of the acid has been neutralised by the base - the mixture has equal quantities of a weak acid and its salt, known as a buffer
- this solution is relatively resistant to change in
on the addition of small amounts of base
the
consider the equilibrium:
as the acid is weak, it can be assumed that very little of it has ionised at equilibrium, so:
it can also be assumed that the salt is fully ionised, so:
substituting:

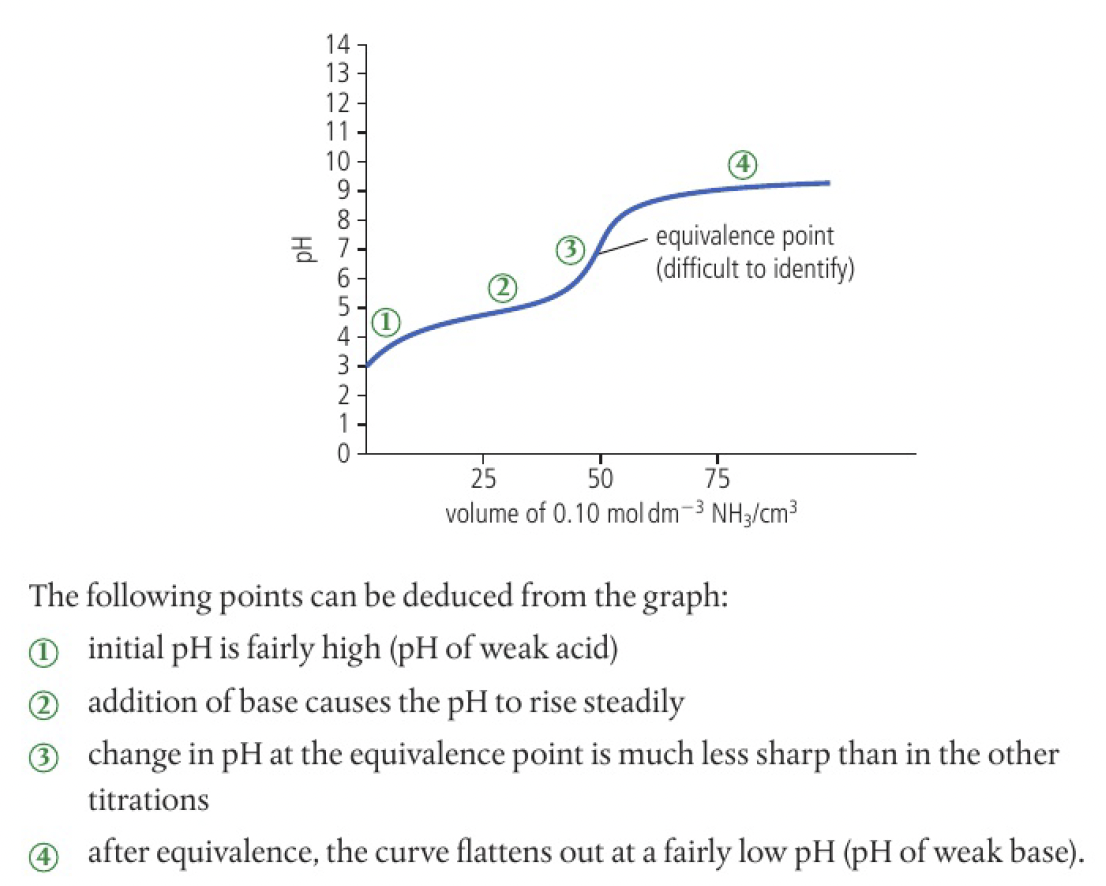
close to the equivalence point, a small addition of titrant causes a significant change in the
challenge questions
- what shape would you expect for a graph of conductivity against volume of acid added in a titration of a strong acid with a strong base? how could this be use to determine the equivalence point?
the conductivity decreases during the neutralisation as the concentration of mobile ions decreases. after equivalence, conductivity increases with the increasing concentration of free ions from the excess acid or base. the minimum conductivity is the equivalence point