chem modelsofparticulatenatureofmatter
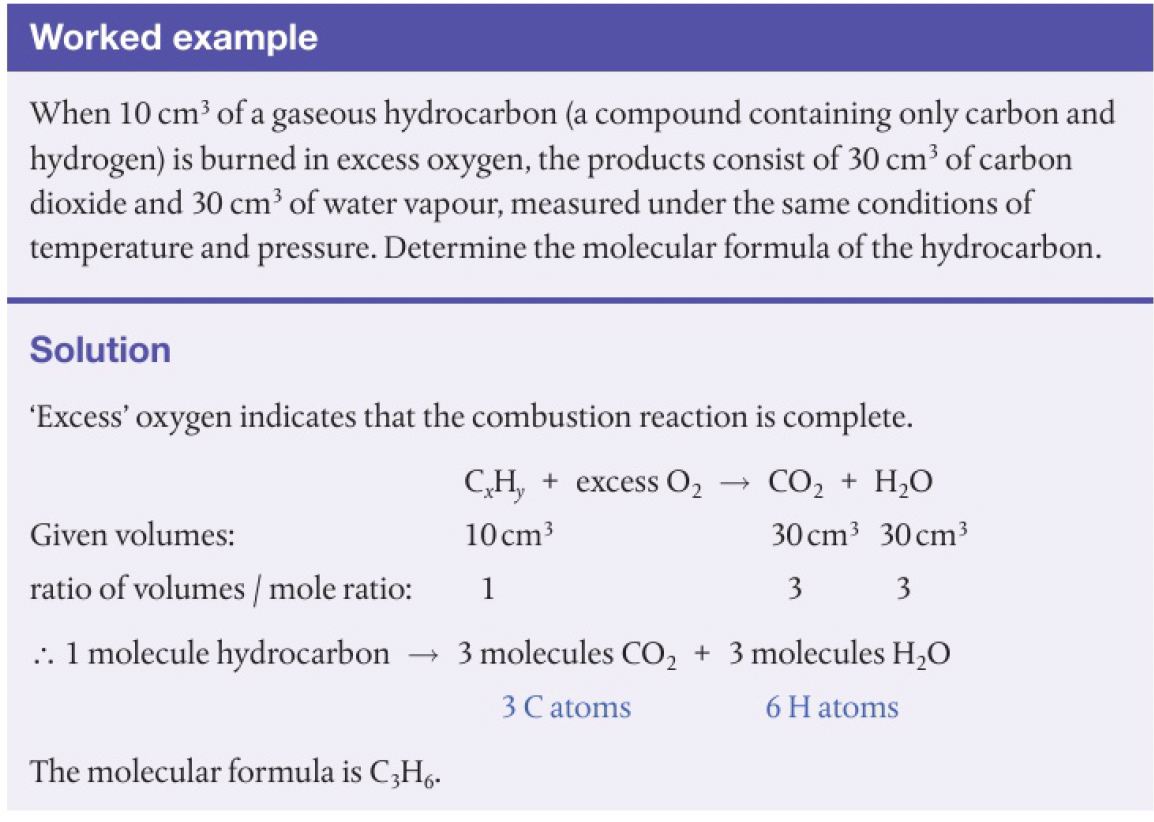
Structure 1.4.6 - Avogadro’s law states that equal volumes of all gases measured under the same conditions of temperature and pressure contain equal numbers of molecules.
this is despite the difference in size and mass of the molecule, are widely spaced out with negligible forces between them, mostly empty space. thus, gas volume is determined only by the number of particles, and by the temperature and pressure.
avogadro’s law is based on the assumptions of the ideal gas model. real gases will deviate from this model when conditions bring the gas closer to forming a liquid - i.e. lower temperature and higher pressure
challenge questions
- Use the explanation above to deduce the chemical equations for the reactions taking place in a deployed airbag.
interesting